Regulation of AcMNPV proliferation by exosomal microRNA in Spodoptera frugiperda Sf9 cells
-
摘要:目的
探究草地贪夜蛾Spodoptera frugiperda Sf9细胞外泌体microRNA(miRNA)对苜蓿银纹夜蛾核多角体病毒(Autographa californica multiple nucleopolyhedrovirus,AcMNPV)增殖的影响。
方法通过不连续蔗糖质量分数梯度超速离心纯化外泌体,随后利用透射电镜观察和纳米颗粒跟踪分析技术对纯化产物进行颗粒直径分析。运用sRNA高通量测序技术筛选AcMNPV感染Sf9细胞后外泌体差异表达的miRNA并预测其潜在靶基因对应的生物学通路。通过转染模拟物(mimic)和病毒感染等细胞试验以及qPCR验证外泌体miRNA的差异表达,并检测差异表达miRNA——sfr-miR-1a-3p对AcMNPV增殖的影响。
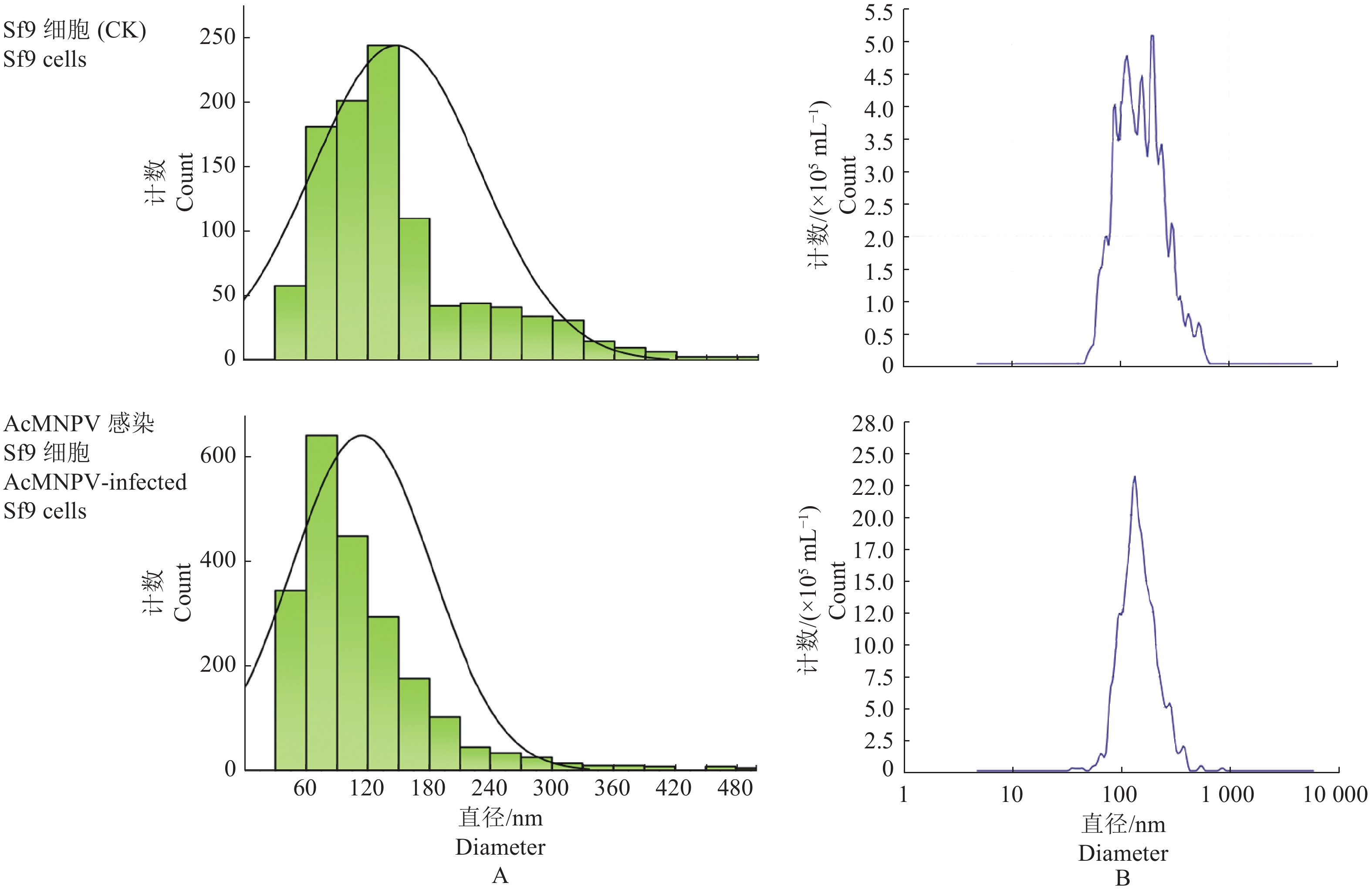
结果成功纯化Sf9细胞外泌体,且AcMNPV感染有效刺激Sf9细胞外泌体的分泌量增加。AcMNPV感染Sf9细胞72 h后,经与Rfam数据库比对,筛选出11个差异表达的外泌体miRNAs,其中,8个miRNAs的转录水平与测序结果趋势一致。通过预测miRNAs靶基因和KEGG富集分析,发现潜在的靶基因主要富集在cAMP信号通路、PI3K-Akt信号通路、癌症通路、人乳头瘤病毒信号通路等,这表明差异表达外泌体miRNA可能参与昆虫天然免疫反应。过表达sfr-miR-1a-3p能显著提高AcMNPV病毒vp39基因的表达。
结论本研究通过sRNA测序筛选了AcMNPV感染后Sf9细胞外泌体的差异表达miRNA,证明了AcMNPV通过促进外泌体分泌,协助被感染细胞传递miRNA−sfr-miR-1a-3p来影响周围未感染细胞,以促进自身增殖。以上结果为昆虫外泌体传递miRNA以调控病毒增殖的机制研究提供了重要的理论依据。
-
关键词:
- 草地贪夜蛾 /
- Sf9细胞 /
- 苜蓿银纹夜蛾核多角体病毒 /
- 外泌体 /
- microRNA /
- sfr-miR-1a-3p
Abstract:ObjectiveTo investigate the effect of Spodoptera frugiperda Sf9 cell exosomal microRNA (miRNA) on proliferation of Autographa californica multiple nucleopolyhedrovirus (AcMNPV).
MethodExosomes were purified using discontinuous sucrose mass fraction gradient ultracentrifugation. Then the purified exosome samples were observed under transmission electron microscopy, and conducted particle diameter analysis using nanoparticle tracking analysis. By sRNA hight throughput sequencing analysis, exosomally differentially expressed miRNAs were identified and biological pathways corresponding to their potential target genes were predicted. Cellular experiments such as mimic transfection and virus infection, as well as qPCR were performed to verify the differential expression of exosomal miRNAs, and detect the effect of the differentially expressed miRNA of sfr-miR-1a-3p on the proliferation of AcMNPV.
ResultExosomes from Sf9 cells were successfully purified, and AcMNPV infection effectively stimulated exosome secretion from Sf9 cells. After 72 h of AcMNPV infection in Sf9 cells, a total of 11 differentially expressed miRNAs were identified in exosomal miRNAs comparing with the Rfam database, in which eight miRNAs’ transcription levels showed consistent trends with the sequencing results. Potential target genes were mainly enriched in the cAMP signaling pathway, PI3K-Akt signaling pathway, cancer pathway, and human papilloma virus signaling pathway, suggesting that differentially expressed exosomal miRNAs might participate in insect innate immune responses. Overexpressing sfr-miR-1a-3p significantly promoted the expression level of the AcMNPV gene vp39.
ConclusionThis study conducts sRNA sequencing to screen differentially expressed miRNAs in AcMNPV-infected Sf9 cell exosomes, demonstrating that AcMNPV promotes infection by enhancing exosome secretion and affecting surrounding uninfected cells via miRNA sfr-miR-1a-3p transfer. This research provides an important theoretical support for understanding the mechanism by which insect exosomal miRNAs regulate virus proliferation.
-
-
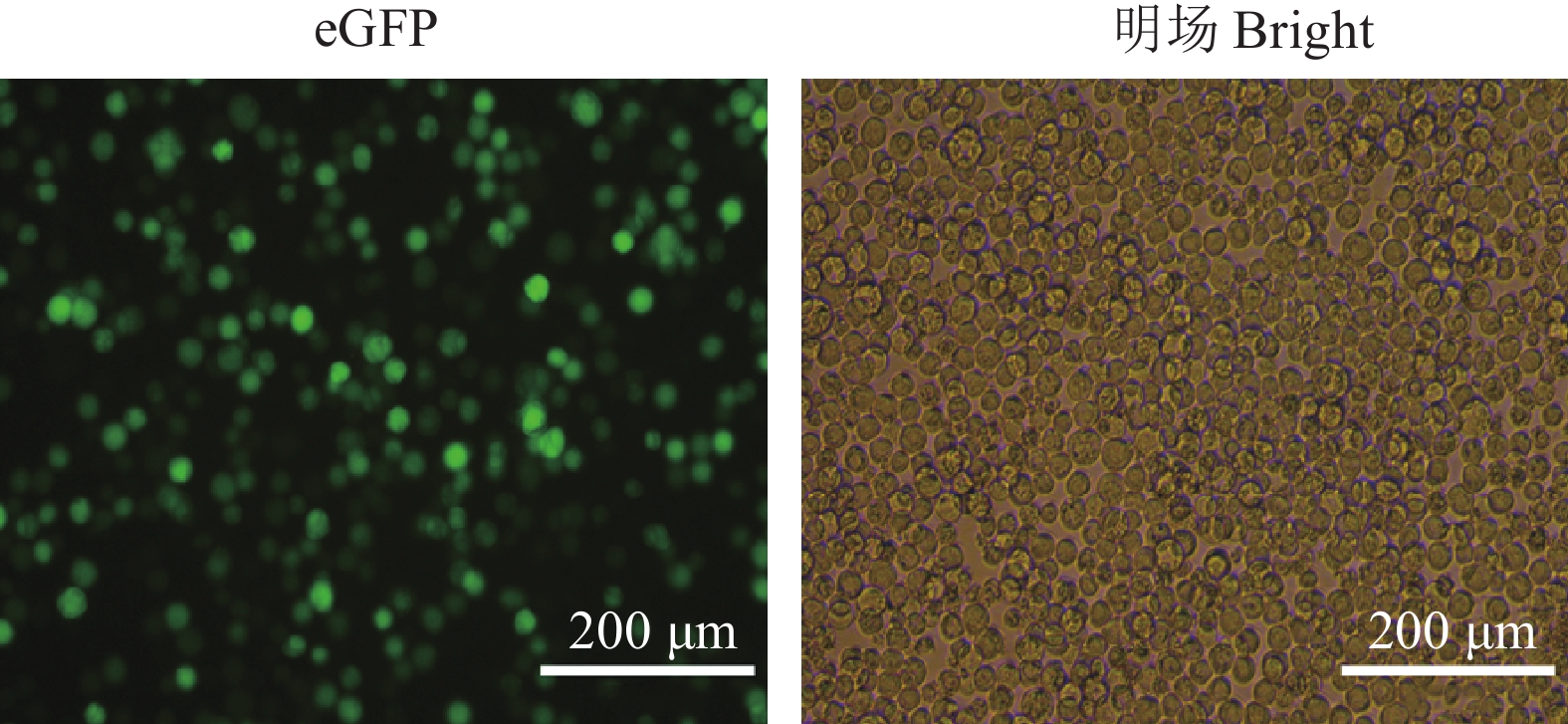
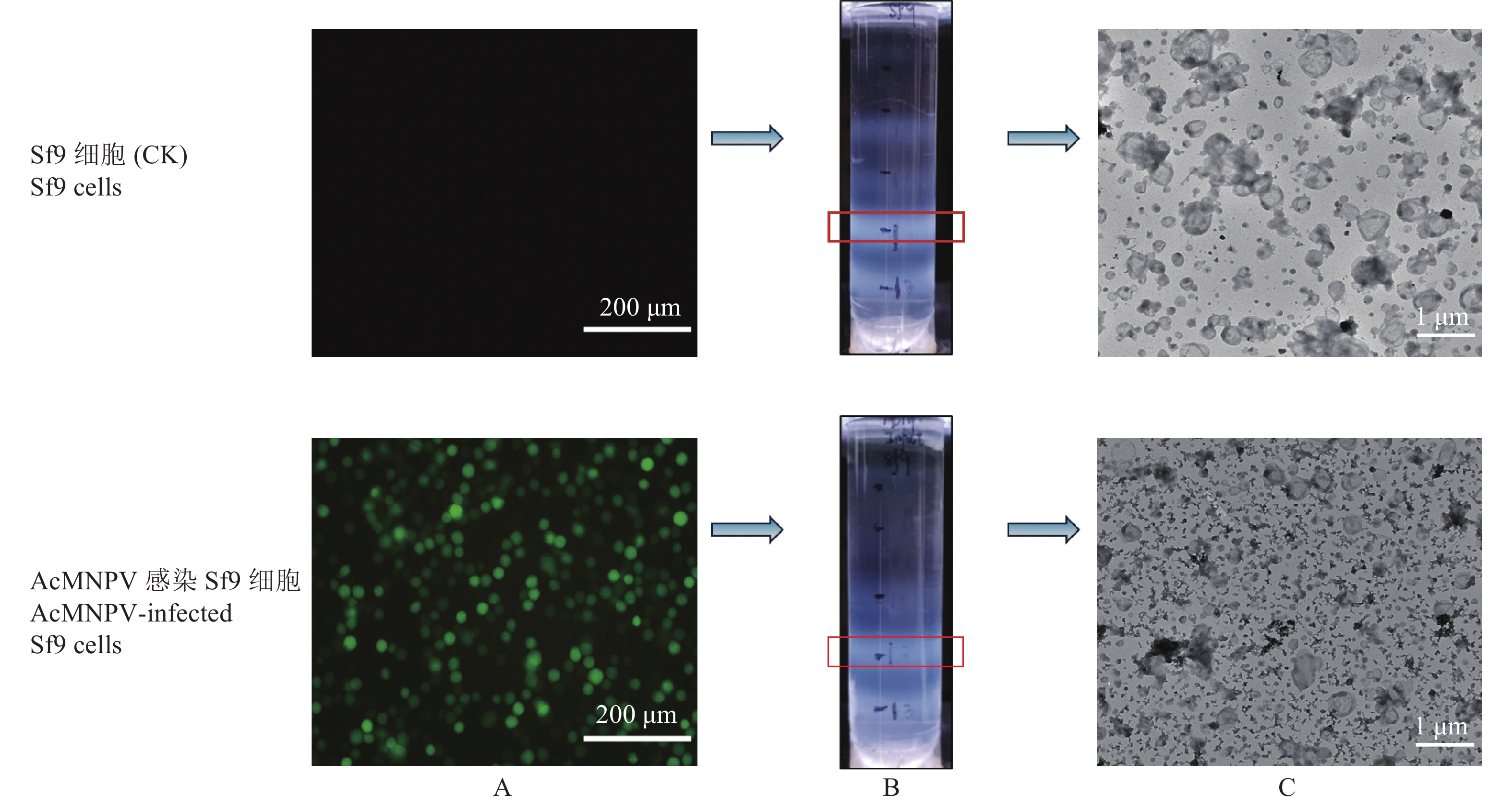
图 3 Sf9细胞外泌体的纯化
A:Sf9细胞和被AcMNPV感染Sf9细胞荧光检测,B:通过蔗糖质量分数梯度离心纯化细胞外泌体,C:纯化产物的透射电镜观察。
Figure 3. Purification of exosomes from Sf9 cells
A: Fluorescence detection of Sf9 cells and Sf9 cells infected by AcMNPV, B: Purification of exosomes by sucrose mass fraction gradient centrifugation, C: Transmission electron microscopy observation of purified product.
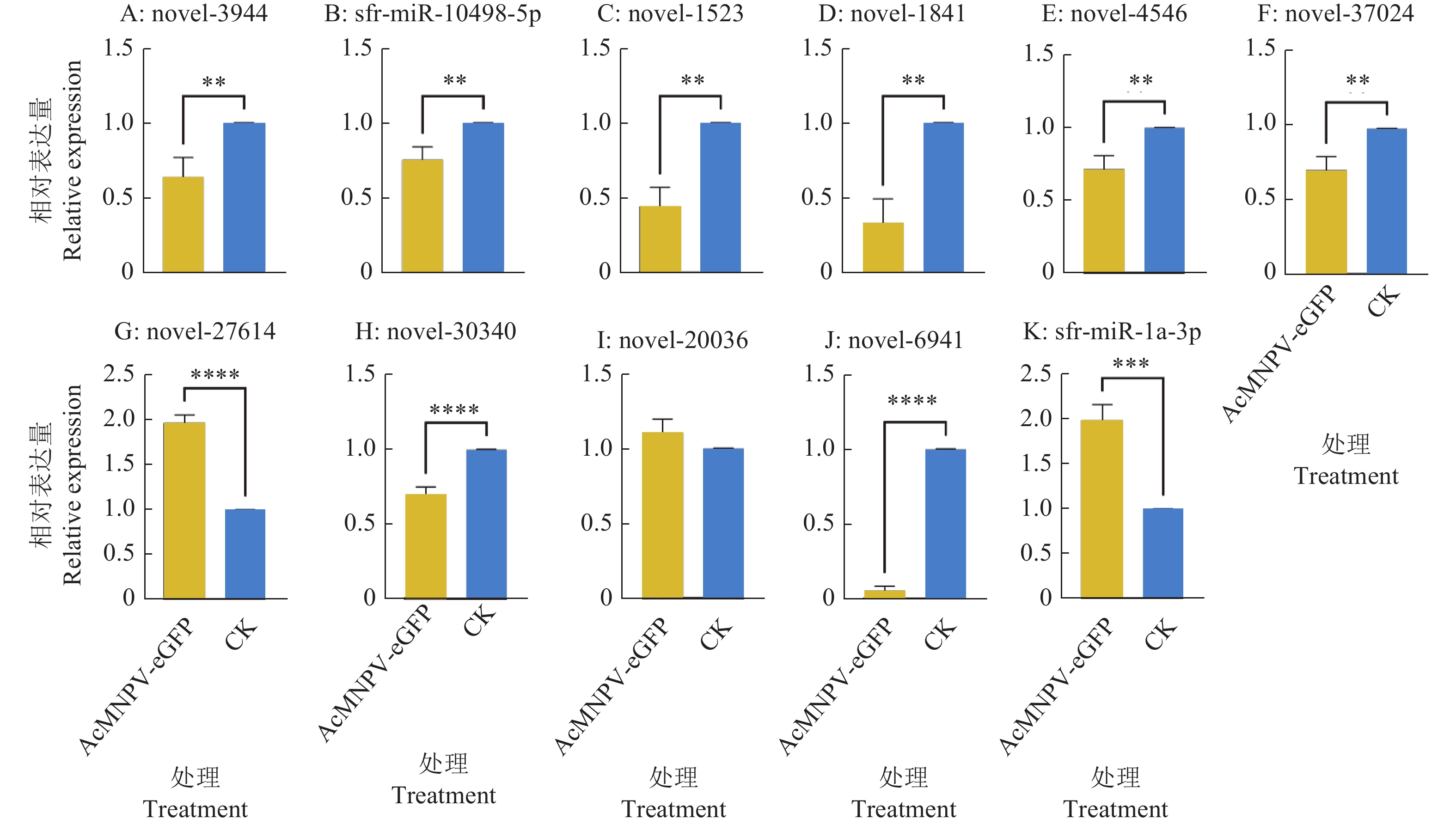
图 5 AcMNPV-eGFP感染Sf9细胞后外泌体差异表达miRNA的验证
“**”“***”“****”分别表示在P<0.01、P<0.001和P<0.000 1水平差异显著(t检验)。
Figure 5. Validation of differentially expressed miRNA in exosomes of Sf9 cells infected by AcMNPV-eGFP
“**” “***” and “****” indicate significant differences at P<0.01, P<0.001 and P<0.000 1 respectively (t test).
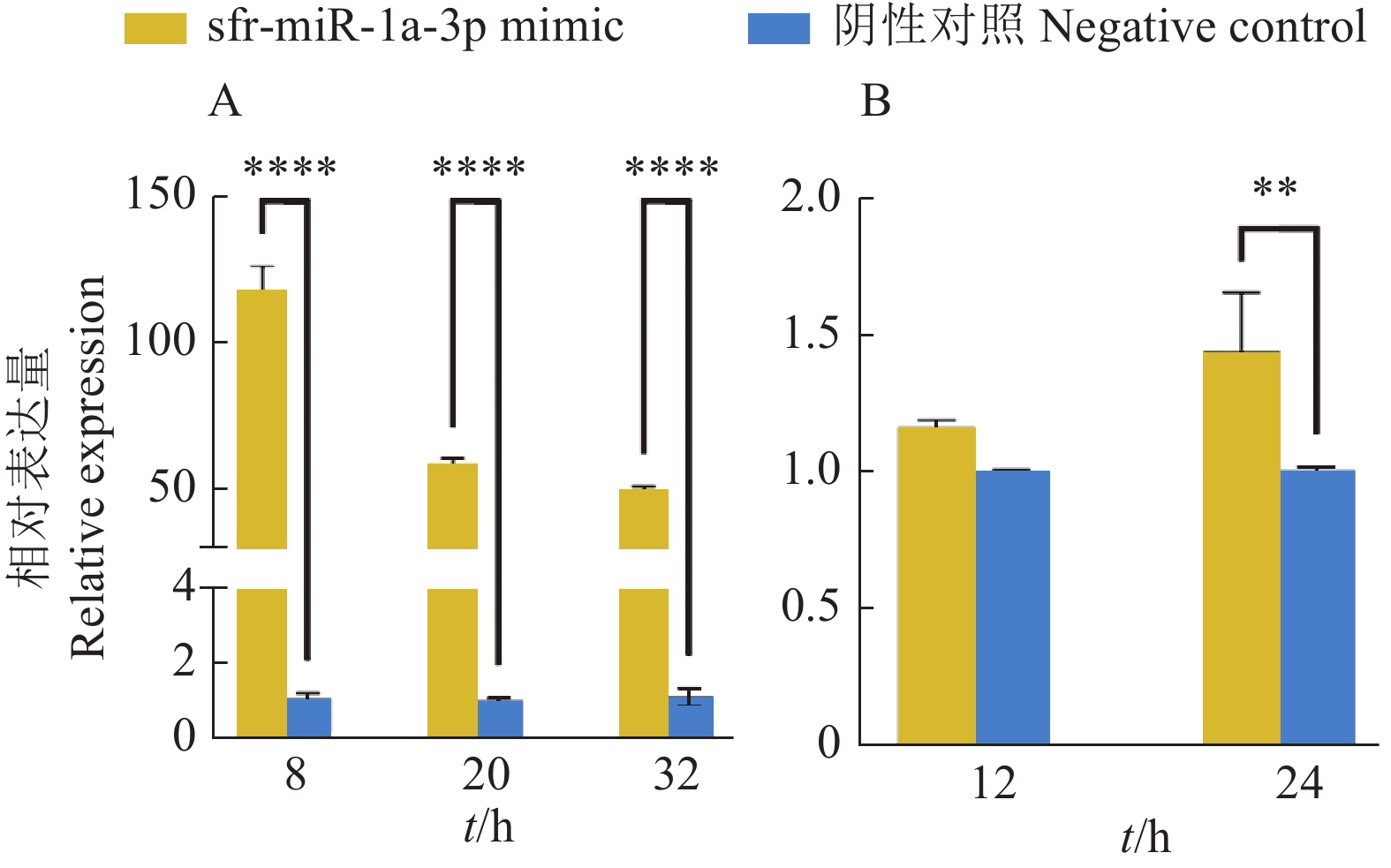
图 6 sfr-miR-1a-3p对AcMNPV增殖的影响
A:sfr-miR-1a-3p 过表达分析,B:vp39在过表达sfr-miR-1a-3p的Sf9细胞中的表达量分析;“**”和“****”分别表示在P<0.01和P<0.000 1水平差异显著(t检验)。
Figure 6. Effect of sfr-miR-1a-3p on proliferation of AcMNPV
A: Overexpression analysis of sfr-miR-1a-3p, B: Analysis of vp39 expression in Sf9 cells overexpressing sfr-miR-1a-3p; “**” and “****” indicate significant differences at P<0.01 and P<0.000 1 respectively (t test).
表 1 本研究所用引物
Table 1 Primers used in this study
引物名称
Primer name引物序列(5′→3′)
Primer sequenceeGFP-Xma I-F AAACCCGGGATGGTGAGCAAGGGCGAGGA eGFP-Kpn I-R AAAGGTACCTTACTTGTACAGCTCGTCCATG novel-27614-qPCR-F GCGGACAATGGTGGCAA novel-30340-qPCR-F GCGTTAGGGAACCGAAGAAA novel-20036-qPCR-F CGAAAAGTCGGTGTGGCTGA novel-6941-qPCR-F CGCGAGCTAAGTCGAAATTTGTA sfr-miR-1a-3p-qPCR-F GCGCGTGGAATGTAAAGAAGT novel-3944-qPCR-F GCGCCATCCCTCACATGAT sfr-miR-10498-5p-qPCR-F CGCGTTGGTCAACGTTCAA novel-1523-qPCR-F CGCGGTCAGGTTGGCC novel-1841-qPCR-F CGCGGATGCGTCGAGTAG novel-37024-qPCR-F GCGCAGCCGAAACTGAAAT novel-4546-qPCR-F GCGCGCTCGTATATTAATTCTC vp39-qPCR-F TGATGCAAGCCGAACAGCTA vp39-qPCR-R GTGTTCGGGTTTGTGGTGTC Sf-GAPDH-qPCR-F TTGCTAACGTCTCGGTCGTC Sf-GAPDH-qPCR-R ATGACACGACCTGTTCCTCG -
[1] 崔志斌, 李智群, 王勇, 等. 草地贪夜蛾研究现状及防控策略[J]. 湖南农业科学, 2020(4): 38-42. [2] 谭心阳, 赵海婷, 曹美伦, 等. 国内草地贪夜蛾发生、为害及防治现状[J]. 现代农药, 2022, 21(5): 13-20. [3] 李颖, 孙兴鲁, 浦冠勤. 昆虫杆状病毒杀虫剂的研究进展[J]. 中国蚕业, 2010, 31(4): 12-14. doi: 10.3969/j.issn.1007-0982.2010.04.003 [4] COCUCCI E, MELDOLESI J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles[J]. Trends in Cell Biology, 2015, 25(6): 364-372. doi: 10.1016/j.tcb.2015.01.004
[5] KALLURI R, LEBLEU V S. The biology, function, and biomedical applications of exosomes[J]. Science, 2020, 367(6478): eaau6977. doi: 10.1126/science.aau6977
[6] THÉRY C, WITWER K W, AIKAWA E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines[J]. Journal of Extracellular Vesicles, 2018, 7(1): 1535750. doi: 10.1080/20013078.2018.1535750
[7] MATHIEU M, MARTIN-JAULAR L, LAVIEU G, et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication[J]. Nature Cell Biology, 2019, 21(1): 9-17. doi: 10.1038/s41556-018-0250-9
[8] KEERTHIKUMAR S, CHISANGA D, ARIYARATNE D, et al. ExoCarta: A web-based compendium of exosomal cargo[J]. Journal of Molecular Biology, 2016, 428(4): 688-692. doi: 10.1016/j.jmb.2015.09.019
[9] PATHAN M, FONSEKA P, CHITTI S V, et al. Vesiclepedia 2019: A compendium of RNA, proteins, lipids and metabolites in extracellular vesicles[J]. Nucleic Acids Research, 2019, 47(D1): D516-D519. doi: 10.1093/nar/gky1029
[10] VAN BALKOM B W M, EISELE A S, PEGTEL D M, et al. Quantitative and qualitative analysis of small RNAs in human endothelial cells and exosomes provides insights into localized RNA processing, degradation and sorting[J]. Journal of Extracellular Vesicles, 2015, 4: 26760. doi: 10.3402/jev.v4.26760
[11] VALADI H, EKSTRÖM K, BOSSIOS A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells[J]. Nature Cell Biology, 2007, 9(6): 654-659. doi: 10.1038/ncb1596
[12] LEE R C, FEINBAUM R L, AMBROS V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14[J]. Cell, 1993, 75(5): 843-854. doi: 10.1016/0092-8674(93)90529-Y
[13] 王娇娇, 王泽英, 罗光彬, 等. 动物microRNA研究现状[J]. 动物医学进展, 2014, 35(6): 144-148. doi: 10.3969/j.issn.1007-5038.2014.06.031 [14] 邓昌鑫, 何倩毓. MicroRNA在昆虫中的研究进展[J]. 黑龙江八一农垦大学学报, 2021, 33(5): 15-21. doi: 10.3969/j.issn.1002-2090.2021.05.003 [15] SINGH C P, SINGH J, NAGARAJU J. A baculovirus-encoded microRNA (miRNA) suppresses its host miRNA biogenesis by regulating the exportin-5 cofactor ran[J]. Journal of Virology, 2012, 86(15): 7867-7879. doi: 10.1128/JVI.00064-12
[16] WU P, SHANG Q, DWETEH O A, et al. Over expression of bmo-miR-2819 suppresses BmNPV replication by regulating the BmNPV ie-1 gene in Bombyx mori[J]. Molecular Immunology, 2019, 109: 134-139. doi: 10.1016/j.molimm.2019.03.013
[17] JIA Q, FU Y. MicroRNA-34-5p encoded by Spodoptera frugiperda regulates the replication and infection of Autographa californica multiple nucleopolyhedrovirus by targeting odv-e66, ac78 and ie2[J]. Pest Management Science, 2022, 78(12): 5379-5389. doi: 10.1002/ps.7160
[18] ZHANG J, ZAFAR J, KONG J, et al. MicroRNA-mediated host immune genes manipulation benefits AcMNPV proliferation in Spodoptera frugiperda[J]. Journal of Agricultural and Food Chemistry, 2023, 71(45): 17175-17187.
[19] HURWITZ S N, NKOSI D, CONLON M M, et al. CD63 regulates epstein-barr virus LMP1 exosomal packaging, enhancement of vesicle production, and noncanonical NF-κB signaling[J]. Journal of Virology, 2017, 91(5). doi: 10.1128/jvi.02251-16.
[20] 徐小洪. 外泌体与新城疫病毒感染的双向调控机制[D]. 长春: 吉林大学, 2019. [21] 高维娟, 许顺江, 丛斌, 等. CCK-8抑制LPS作用下大鼠肺间质巨噬细胞NF-κB活性的cAMP-PKA通路研究[J]. 中国病理生理杂志, 2006, 22(10): 1891-1895. [22] JIANG L. Insights into the antiviral pathways of the silkworm Bombyx mori[J]. Frontiers in Immunology, 2021, 12: 639092. doi: 10.3389/fimmu.2021.639092
[23] 刘小民, 袁明龙. 昆虫天然免疫相关基因研究进展[J]. 遗传, 2018, 40(6): 451-466. [24] DATTA S R, BRUNET A, GREENBERG M E. Cellular survival: A play in three Akts[J]. Genes & Development, 1999, 13(22): 2905-2927.
[25] YAO R, COOPER G M. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor[J]. Science, 1995, 267(5206): 2003-2006. doi: 10.1126/science.7701324
[26] 钟佳琳, 郑立, 贺花, 等. PI3K/Akt信号通路相关的生物学调控机制研究进展[J]. 基因组学与应用生物学, 2019, 38(1): 143-147. [27] XIAO W, YANG Y, WENG Q, et al. The role of the PI3K-Akt signal transduction pathway in Autographa californica multiple nucleopolyhedrovirus infection of Spodoptera frugiperda cells[J]. Virology, 2009, 391(1): 83-89. doi: 10.1016/j.virol.2009.06.007
[28] WU P, JIANG X, SANG Q, et al. Inhibition of miR-274-3p increases BmCPV replication by regulating the expression of BmCPV NS5 gene in Bombyx mori[J]. Virus Genes, 2017, 53(4): 643-649. doi: 10.1007/s11262-017-1466-7
[29] DUBEY S K, SHRINET J, JAIN J, et al. Aedes aegypti microRNA miR-2b regulates ubiquitin-related modifier to control chikungunya virus replication[J]. Scientific Reports, 2017, 7: 17666. doi: 10.1038/s41598-017-18043-0
[30] WU P, JIANG X, GUO X, et al. Genome-wide analysis of differentially expressed microRNA in Bombyx mori infected with nucleopolyhedrosis virus[J]. PLoS One, 2016, 11(11): e0165865. doi: 10.1371/journal.pone.0165865



 下载:
下载: