Cloning and function analysis of starch synthase SSⅡa promoter in maize
-
摘要:目的
克隆玉米Zea mays淀粉合成酶SSⅡa启动子,并分析其功能,为进一步研究和应用SSⅡa启动子奠定基础。
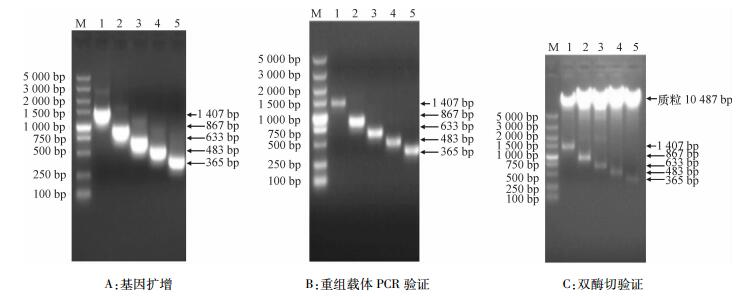
方法通过NCBI上公布的玉米基因组序列,在网站MaizeGDB上BLAST查找到SSⅡa 5′侧翼序列,利用PCR方法从玉米B73中克隆SSⅡa启动子;通过PlantCare在线分析启动子顺式作用元件,用特异性引物分别克隆出长度为1 407、867、633、483和365 bp的片段,与植物表达载体pCAMBIA3301连接,构建5种5′缺失体的植物表达载体,命名为P1、P2、P3、P4和P5。用农杆菌介导法转化拟南芥Arabidopsis thaliana,获得转基因拟南芥。
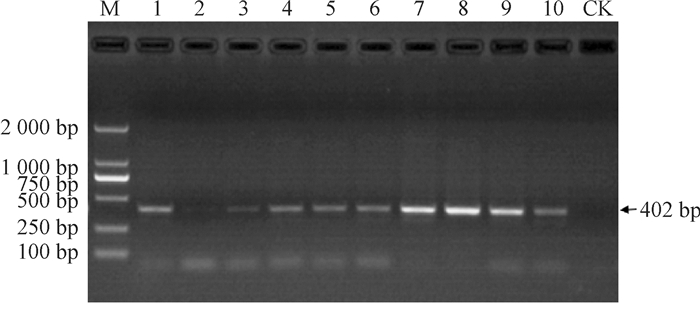
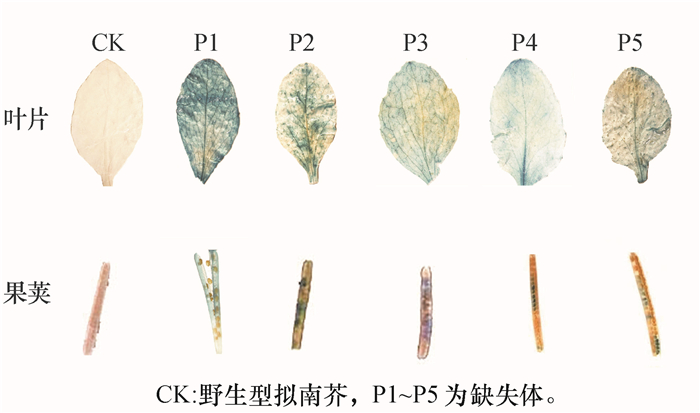
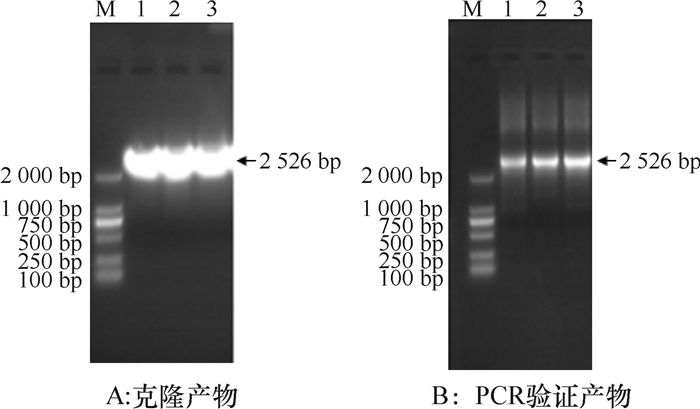
结果以玉米B73基因组DNA为模板,用特异性引物SSⅡaF/SSⅡaR进行扩增,得到2 526 bp序列;除草剂筛选的阳性拟南芥植株PCR验证均检测出gus基因;GUS组织化学分析表明,5种类型启动子构建的表达载体在成熟期叶片、果荚中均显蓝色;gus基因定量分析表明,成熟期5种转基因拟南芥叶片中, gus基因表达量P1最高,其他基本一致;种子中gus基因表达量P1和P2相近,且高于P3、P4和P5。
结论成功克隆玉米SSⅡa启动子;构建的5种SSⅡa启动子缺失体表达载体在转基因拟南芥中均具有活性,长度为1 407 bp(P1)和867 bp(P2)的启动子具有胚乳特异性。
Abstract:ObjectiveTo clone maize (Zea mays) starch synthase SSⅡa promoter, analyze its function, and provide a basis for its future research and application.
MethodThe SSⅡa 5′ flanking sequence was found on Maize GDB by BLASTing the maize genome sequence published on NCBI, and the SSⅡa promoter was cloned from maize B73 using PCR. We analyzed the cis elements of the promoter using PlantCare. Fragments of 1 407, 867, 633, 483, and 365 bp were cloned with specific primers, and were inserted into the plant expression vector pCAMBIA3301, respectively. Five plant expression vectors with different 5′ deletions of the SSⅡa promoter were constructed and named P1, P2, P3, P4 and P5.The transgenic Arabidopsis thaliana plants were obtained through Agrobacterium-mediated transformation.
ResultA DNA fragment of 2 526 bp was obtained by PCR amplification with maize B73 genome DNA as template and SSⅡaF/SSⅡaR as specific primers. The positive A.thaliana plants, which were screened by herbicide, had gus gene by PCR detection. The histochemical analysis of GUS showed that the expression vectors of five promoters were blue in leaves and pods at maturity. The quantitative analysis of gus gene showed that among five transgenic A.thaliana at maturity, the expression level of P1 in leaves was the highest and the others were basically the same, and the expression levels of P1 and P2 in seeds were similar, both being higher than those of P3, P4 and P5.
ConclusionThe maize SSⅡa promoter has been successfully cloned. The five constructed expression vectors with different 5′ deletions of the SSⅡa promoter all have activities in transgenic A.thaliana, and the promoters with the length of 1 407 bp (P1) and 867 bp (P2) have endosperm specificity.
-
Keywords:
- maize /
- starch synthesis enzyme /
- SSⅡa promoter /
- cloning /
- vector construction /
- function analysis
-
-
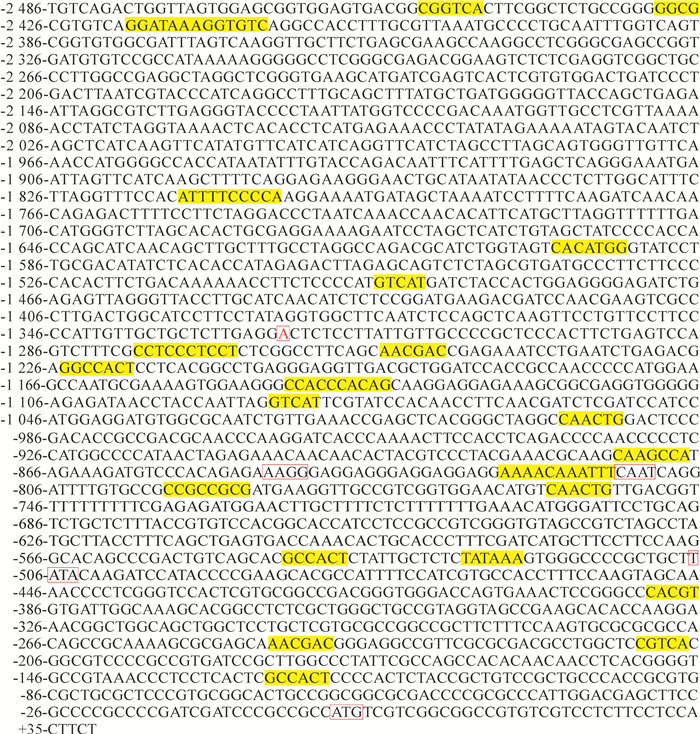
表 1 SSⅡa启动子区顺式作用元件
Table 1 Cis-acting elements of SSⅡa promoter
元件名称 序列 元件功能 元件位置 CAAT-box CAATT、CAAAT、CAAT 启动子和增强子区常见的顺式作用元件 -2 380、-1 094、-2 088、-1 165、-1 377、-1 166、-1 095、-1 915、-2 013、-1 032、-815 CAT-box GCCACT 与分生组织表达相关的作用元件 -1 225、-546、-127 CE3 GACGCGTGTC 参与ABA和VP1响应的作用元件 -2 430 CG-motif CCATGGGG 光响应元件 -1 945 G-box CACATGG 光响应元件 -1 601、-392 I-box GGATAAGGTG 光响应元件 -2 419 MBS CGGTCA MYB结合位点 -2 452 Skn-1-motif GTCAT 胚乳表达所需的作用元件 -1 497、-1 087 GCN4-motif CAAGCCA 胚乳表达调控作用元件 -875 Sp1 CC(G/A)CCC 光响应元件 -1 279、-1 145 TATA-box TAATA、TATA、ATATAA、 核心启动子元件(在转录起始位点上游) -1 850、-2 028、-1 389、-1 847、-1 931、-1 848、-2 030、-529、-508 TC-rich repeats ATTTTCTCCA 参与防御和应激反应的作用元件 -1 815 TGA-element AACGAC 生长素响应的作用元件 -1 256、-247 TGACG-motif TGACG 参与茉莉酸甲酯响应的作用元件 -2 458、-1 198、-754 ABRE CACGTG 参与脱落酸响应的元件 -392 CGTCA-motif CGTCA 参与茉莉酸甲酯响应的作用元件 -213 GARE-motif TCTGTTG 赤霉素应答作用元件 -1 029 HSE AAAAAATTTC 参与热胁迫应答的作用元件 -824 MBS CAACTG 参与抗旱诱导的MYB结合位点 -1 000、-761 O2-site GATGATGTGG 参与玉米醇溶蛋白代谢调控的作用元件 -1 044 motif IIb CCGCCGCGCT 脱落酸响应元件 -796 -
[1] MÜLLER-RÖBER B T, KOβMANN J, HANNAH L C, et al. One of two different ADP-glucose Pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose[J]. Mol Gen Genet, 1990, 224(1): 136-146. doi: 10.1007/BF00259460
[2] NAOMI S S, ICHIRO M. Constitutive promoters available for trans-gene expression instead of CaMV35S RNA promoter: Arabidopsis promoters of tryptophan synthase protein subunit and phytochrome B[J]. Plant Biotechnol-nar, 2002, 19(1): 19-26. doi: 10.5511/plantbiotechnology.19.19
[3] 覃鸿妮, 蔡一林, 孙海燕, 等.种植密度对不同株型玉米蔗糖代谢和淀粉合成相关酶活性的影响[J].中国生态农业学报, 2010, 18(6):1183-1188. http://www.cnki.com.cn/Article/CJFDTOTAL-ZGTN201006007.htm [4] 崔喜艳, 胡广宇, 孙小杰, 等.1个新的玉米半透明皱缩胚乳突变体的遗传分析及基因定位[J].华南农业大学学报, 2014, 35(5) :31-35. doi: 10.7671/j.issn.1001-411X.2014.05.006 [5] 张军杰, 黄玉碧.玉米可溶性淀粉合成酶研究进展[J].玉米科学, 2006, 14(6):151-154. http://youxian.cnki.com.cn/yxdetail.aspx?filename=FZZW20170418001&dbname=CAPJ2015 [6] 王振华, 亢伟民, 张新.高淀粉玉米及其开发利用[J].玉米科学, 2002, 10(3):90-92. http://www.cnki.com.cn/Article/CJFDTOTAL-YMKX200203033.htm [7] 彭泽斌, 田志国.高淀粉玉米的产业化潜力分析[J].作物杂志, 2003(6):10-12. http://www.cnki.com.cn/Article/CJFDTOTAL-ZWZZ200306003.htm [8] HARN C, KNIGHT M, RANAKRISHNAN A, et al. Isolation and characterization of the zSSIIa and zSSIIb starch synthase cDNA clones from maize endosperm[J]. Plant Mol Biol, 1998, 37(4): 639-649. doi: 10.1023/A:1006079009072
[9] KNIGHT M E, HARN C, LILLEY C E, et al. Molecular cloning of starch synthase I from maize (W64) endosperm and expression in Escherichia coli [J]. Plant J, 1998, 14(5): 613-622. doi: 10.1046/j.1365-313X.1998.00150.x
[10] GAO M, WANAT J, STINARD P S, et al. Characterization of dull1, a maize gene coding for a novel starch synthase[J]. Plant Cell, 1998, 10(3): 399-412. doi: 10.1105/tpc.10.3.399
[11] BLAUTH S L, YAO Y, KLUCINEC J D, et al. Identification of mutator insertional mutants of starch-branching enzyme 2a in corn[J]. Plant Physiol, 2001, 125(3): 1396-1405. doi: 10.1104/pp.125.3.1396
[12] 相恒佐. 毛百合GPAT基因启动子的克隆和功能鉴定[D]. 沈阳: 沈阳农业大学, 2014: 15-16. [13] 佟珊珊. 根特异性启动子表达AAP1-bar双价基因在玉米新材料创制中的应用研究[D]. 长春: 吉林农业大学, 2014. [14] JEFERSON G, RLCARDO J.S, ARTHUR G F, et al. Iron homeostasis related genes in rice[J].Genet Mol Biol, 2003, 26(4): 477-497. doi: 10.1590/S1415-47572003000400012
[15] THOMAS D S, KENNETH J L. Analyzing real-time PCR data by the comparative CT method[J].Nature, 2008, 3(6): 1101-1108.
[16] 崔喜艳, 陈众峰, 范贝, 等.栽培大豆rbcS启动子的克隆及在转基因烟草中功能缺失分析[J]西北农林科技大学学报, 2015, 43(5): 114-121, 128. http://www.cnki.com.cn/Article/CJFDTOTAL-XBNY201505018.htm [17] 曾礼华, 汪瀚宇, 谢程程, 等.玉米淀粉合成酶基因GBSS启动子的克隆与鉴定[J].植物生理学报, 2015, 51 (9): 1433-1439. http://www.cnki.com.cn/Article/CJFDTOTAL-ZWSL201509012.htm [18] 任红丽, 黄玉碧. 玉米淀粉合成酶Ⅰ基因(zsS1)启动子载体构建及胚乳瞬时表达[D]. 雅安: 四川农业大学, 2005. [19] HU Y F, LI Y P, ZHANG J, et al.PzsS3a, a novel endosperm specific promoter from maize (Zea mays L.) induced by ABA[J]. Biotechnol Lett, 2011, 33(7):1465-1471. doi: 10.1007/s10529-011-0582-z
[20] LI Q F, SUN S S, LIU Q Q. Characterization of the spatial and temporal expression of the OsSSII-3 gene encoding a key soluble starch synthase in rice[J].J Sci Food Agr, 2013, 93(13):3184-3190. doi: 10.1002/jsfa.2013.93.issue-13



 下载:
下载: