Bioinformatics analysis and prokaryotic expression of apical membrane antigen protein AMA1 of Isospora suis
-
摘要:目的
预测分析猪等孢球虫顶膜抗原蛋白AMA1的生物学特性、结构及功能,并构建AMA1基因的原核表达载体,表达、纯化AMA1蛋白。
方法本研究从NCBI数据库中获得猪等孢球虫AMA1基因序列,使用相关生物信息学预测工具对AMA1基因编码的蛋白进行分析。构建原核表达载体pET23a-AMA1,并将其转入至大肠埃希菌表达菌株BL21 (DE3)中,对诱导时间、温度及IPTG浓度进行优化,确定最佳诱导表达条件。采用镍柱亲和层析法进行蛋白纯化,获得AMA1重组蛋白并进行SDS-PAGE和Western blot鉴定分析。
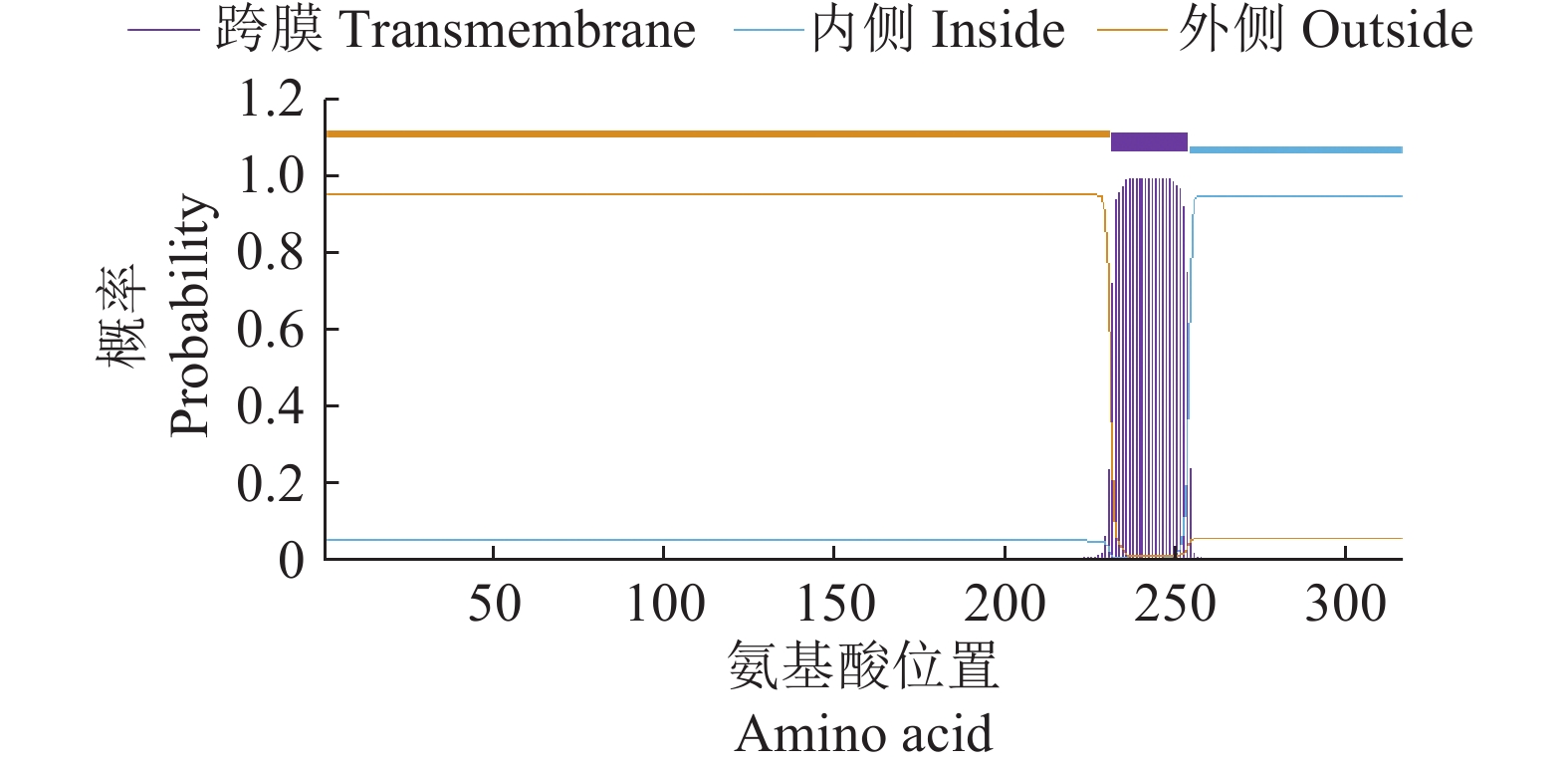
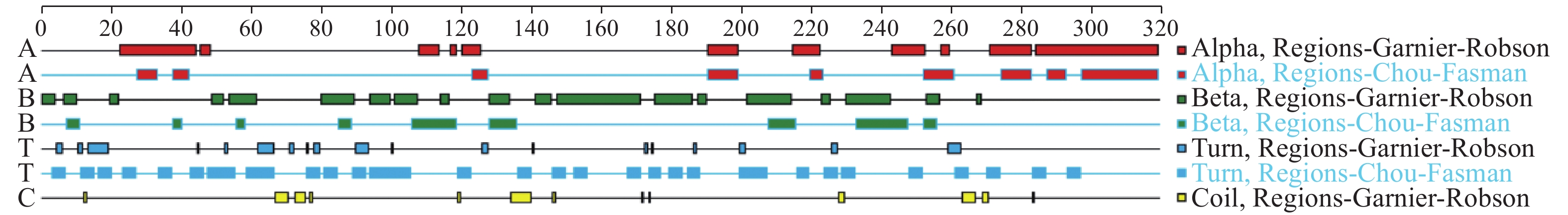
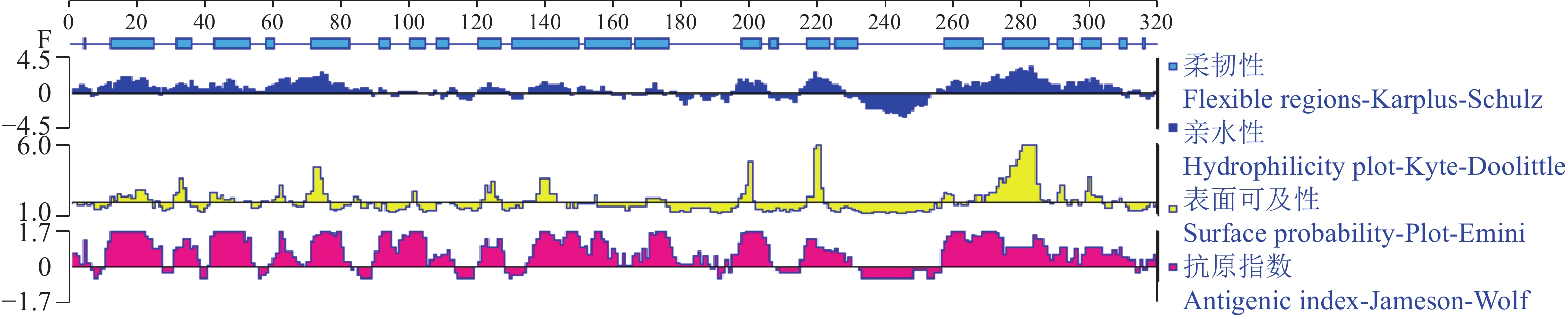
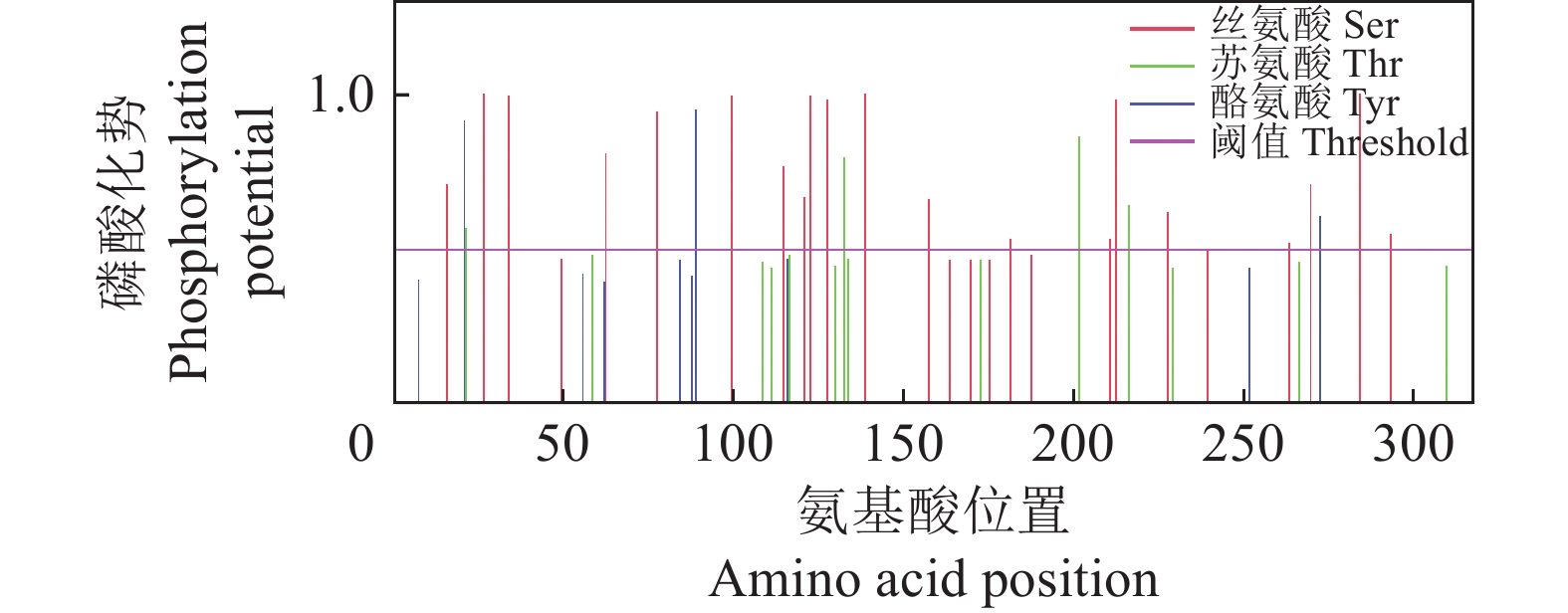
结果生物信息学预测显示,AMA1蛋白由317个氨基酸组成,蛋白分子式为C1512H2310N394O490S14,是不稳定的亲水性蛋白;其二级结构α螺旋占17.74%,β折叠占30.65%,转角占30.65%,无规则卷曲占20.96%;属于无信号肽的跨膜蛋白,具有5个B细胞抗原表位。成功构建pET23a-AMA1重组表达质粒,经诱导表达条件优化后,确定最佳诱导表达条件为0.2 mmol/L IPTG浓度下于28 ℃诱导12 h,主要以可溶性蛋白形式存在,蛋白相对分子质量约为25 800,纯化蛋白质量浓度为0.25 mg/mL。
结论阐明了猪等孢球虫AMA1蛋白的结构特征,通过原核诱导表达获得了重组蛋白,为建立猪等孢球虫病的免疫学诊断方法奠定了基础,并为后续疫苗的研发提供了新的候选基因。
Abstract:ObjectiveTo predict and analyze the biological characteristics, structure and function of the apical membrane antigen 1 (AMA1) in Isospora suis, as well as construct the prokaryotic expression vector of AMA1 gene to express and purify AMA1 protein.
MethodThe AMA1 gene sequence of I. suis was obtained from NCBI database, and the protein encoded by AMA1 gene was analyzed using relevant bioinformatics prediction tools. The recombinant prokaryotic expression vector pET23a-AMA1 was constructed and transferred into the expression strain BL21 (DE3) of Escherichia coli. The induction time, temperature and IPTG concentration were optimized to determine the optimal induction expression conditions. The recombinant AMA1 protein was purified by nickel column affinity chromatography and identified by SDS-PAGE and Western blot.
ResultBioinformatics prediction showed that AMA1 protein was composed of 317 amino acids, and its molecular formula was C1512H2310N394O490S14, which was an unstable hydrophilic protein. The secondary structure prediction of the AMA1 protein yielded a profile consisting of 17.74% α helix, 30.65% β folding, 30.65% rotation, and 20.96% random coil. It was a transmembrane protein without signal peptide and had five B cell epitopes. At the same time, pET23a-AMA1 recombinant expression plasmid was successfully constructed, and the optimal induction expression condition was 0.2 mmol/L IPTG inducing for 12 h at 28 ℃, which mainly existed in the form of soluble protein. The relative molecular mass of recombinant protein was about 25 800, and the mass concentration of purified protein was 0.25 mg/mL.
ConclusionThe results of this study elucidate the structural characteristics of AMA1 protein of I. suis and the recombinant protein is obtained through prokaryotic induction expression, which lays a foundation for the establishment of immunological diagnosis of I. suis and provides a new candidate gene for the development of subsequent vaccines.
-
Keywords:
- Isospora suis /
- Apical membrane antigen /
- AMA1 /
- Prokaryotic expression /
- Bioinformatics
-
草地贪夜蛾Spodoptera frugiperda J. E. Smith起源于美洲热带和亚热带地区,是典型的迁飞性害虫[1],其幼虫可取食玉米、水稻、高粱和甘蔗等350多种植物[2-3]。草地贪夜蛾于2019年1月入侵我国云南[4-7],截至2019年7月23日,已经蔓延到我国21个省(市、自治区),严重威胁我国农业及作物生产[8]。自草地贪夜蛾侵入我国以来,传播迅速,且我国大部分地区均适宜草地贪夜蛾生存,如果不进行人为干预,其势必会对我国作物安全造成重大影响[9]。化学农药对草地贪夜蛾的防治已有几十年的应用历史[10],目前许多国家的田间种群对多数传统农药已产生了不同程度的抗性[11],靶标害虫产生抗性使得农药的使用剂量加大,而农药使用剂量的增加会引发农产品质量安全、环境安全和生物多样性问题[12-14]。近年来,世界各国都在大力推广和使用生物农药来防治害虫,生物农药防治效果独特,具有多重作用机制,不易引起抗药性,而且对环境安全[15]。印楝素是一种从印楝中提取出来的生物活性物质,是世界公认的广谱、高效、低毒、易降解、无残留的杀虫剂[16-17]。许多研究表明,印楝素对多种害虫具有拒食、触杀、胃毒、抑制生长发育和影响卵巢发育的作用[18]。本文测定了室内0.3%(φ)印楝素乳油和40%(w)印楝素干粉对草地贪夜蛾毒杀活性和拒食活性以及0.3%印楝素乳油对草地贪夜蛾的田间防效,旨在为科学防治草地贪夜蛾提供参考和依据。
1. 材料与方法
1.1 材料
1.1.1 供试虫源
2019年5月在广州市花都区花山镇紫西村玉米地(113°26′50.43″N,23°48′66.63″E)采集草地贪夜蛾卵块,因1龄和2龄幼虫取食叶片后会留下一层膜,3龄及高龄幼虫可以把叶片吃穿,1龄幼虫取食量较小,试验叶片可能无法满足高龄幼虫的取食量,故卵孵化后,以玉米嫩叶饲养至2龄和3龄幼虫供试。饲养条件为温度25 ℃ ± 2 ℃、湿度为60% ± 5%、光周期为16 h光∶8 h暗。
1.1.2 供试玉米
供试玉米种植地为广州市花都区花山镇紫西村,试验时玉米处于喇叭口期,植株长势均匀。试验前及试验过程中均未喷施其他农药,玉米栽培及管理条件符合当地的农业实践管理。
1.1.3 供试药剂
0.3%印楝素乳油,成都绿金生物科技有限责任公司;40%印楝素干粉,华南农业大学天然农药与化学生物学教育部重点实验室。
1.1.4 试验仪器及工具
电动喷雾器、叶面积测定仪、定量药匙、移液枪、培养皿、天平、水桶、量杯、滤纸、剪刀、毛刷等。
1.2 方法
1.2.1 室内毒力试验
采用浸叶法[19]测定了40%印楝素干粉对草地贪夜蛾2龄和3龄幼虫的毒力,根据预备试验结果,将40%印楝素干粉用φ为70%的丙酮溶液配制成0.1、0.5、1.0和5.0 mg/L的 4个印楝素质量浓度。将洁净的新鲜玉米嫩叶剪成3 cm×5 cm的叶片,浸渍药液3 s,自然晾干后置于垫有滤纸(直径9 cm)保湿的培养皿中,再选取大小一致的2龄幼虫20头饥饿处理4 h后接入培养皿内,3龄幼虫每皿只接入1头,每处理测定20头,每处理重复3次。以φ为70%的丙酮溶液浸渍3 s后自然晾干的嫩叶喂食为对照。
将0.3%印楝素乳油用水稀释成0.1、0.5、1.0和5.0 mg/L的 4个印楝素质量浓度,以清水为对照,均匀喷洒至盆栽玉米叶片上,待其晾干后,选取新鲜玉米嫩叶剪成3 cm×5 cm的叶片,置于垫有滤纸(直径9cm)保湿的培养皿中,再选取大小一致的2龄幼虫20头饥饿处理4 h后接入培养皿内,3龄幼虫每皿只接入1头,每处理测定20头、重复3次。
处理后1、2和3 d用叶面积测定仪测定取食面积,并根据以下公式计算拒食率:
拒食率 = (对照组取食叶面积−处理组取食叶面积)/对照组取食叶面积×100%。
并使用Excel进行毒力回归计算,得到毒力回归方程、AFC50、上下限及相关系数。
处理后1、2、3、5和7 d检查幼虫的存活状态,以毛刷轻触幼虫体表,无反应判定为死亡,记录死亡数,按照以下公式计算各处理幼虫的死亡率和校正死亡率:
死亡率 = 死虫数/供试虫数 × 100%,
校正死亡率 = (处理组死亡率−对照组死亡率)/(100−对照组死亡率)× 100%。
使用Excel进行毒力回归计算,得到毒力回归方程、LC50、上下限及相关系数,采用GraphPad Prism 5作图,SPSS19.0数据处理软件进行差异显著性分析。
1.2.2 田间药效试验
田间药效试验于2019年6月17日至6月24日在广东省广州市花都区花山镇紫西村玉米地进行,草地贪夜蛾为害期为幼虫各个阶段,试验期间玉米正处于喇叭口期,试验地玉米52 500株/hm2。试验共设3个处理浓度,即用水将0.3%印楝素乳油稀释500、800和1 000倍,稀释倍数越高,药剂浓度越低。每个处理设置3个重复小区,每个小区30 m2,各小区之间设置1 m宽的隔离带,另设对照小区3个,各30 m2。试验用药时天气多云,施药当天及施药后3 d无降雨。
将0.3%印楝素乳油稀释后,对玉米进行全株均匀喷雾施药,制剂用药量为750 mL/hm2。对照小区使用清水按照相同方法处理。
在每个小区中采用“Z”字型六点取样法,每点5株,共计30株,试验前做好标记并统计玉米上的虫口数量,施药后1、3 和7 d按照相同方式调查虫口数量,并同时观察小区玉米生长情况及药害发生情况。按照以下公式计算虫口减退率和防治效果,并用SPSS19.0数据处理软件进行差异显著性分析。
虫口减退率 = (药前活虫数−药后活虫数)/ 药前活虫数 × 100%,
防治效果 = (处理区虫口减退率−对照组虫口减退率)/(1−对照组虫口减退率)× 100%。
2. 结果与分析
2.1 印楝素对草地贪夜蛾幼虫的室内毒杀活性
为评价使用印楝素对草地贪夜蛾的毒杀活性与防效,在室内测定了0.3%印楝素乳油和40%印楝素干粉对草地贪夜蛾幼虫的毒杀活性,结果见图1和表1。从图1可以看出,质量浓度为5 mg/L的印楝素对草地贪夜蛾2龄和3龄幼虫具有良好的毒杀活性。在质量浓度为5 mg/L时,0.3%印楝素乳油处理草地贪夜蛾后1、2、3、5和7 d,2龄幼虫的死亡率分别为6.67%、23.33%、53.33%、70.00%和86.67%,3龄幼虫的死亡率分别为8.33%、26.67%、60.00%、80.00%和90.00%;40%印楝素干粉处理草地贪夜蛾后1、2、3、5和7 d,2龄幼虫的死亡率分别为1.67%、16.67%、50.00%、61.67%和75.00%,3龄幼虫的死亡率分别为6.67%、20.00%、53.33%、65.00%和83.33%。经Tukey法差异显著性分析,在P<0.05水平上,7 d后处理组与对照组的死亡率差异显著,处理组之间无显著差异,对照组之间无显著差异。试验发现,印楝素对草地贪夜蛾的毒杀效果较为缓慢,未死亡的幼虫出现行动迟缓、躯体僵硬等现象。
表 1 印楝素对草地贪夜蛾2龄和3龄幼虫的毒力分析Table 1. The toxicity analyses of azadirachtin on the 2nd and 3 rd instar larvae of Spodoptera frugiperda幼虫
Larva处理
Treatmentt/d 毒力回归方程1)
Regression equationLC50/(mg·L−1) 95%置信区间
95% confidence limit相关系数(r)
Correlation coefficient2龄
2nd instar0.3%印楝素乳油
0.3% Azadira-
chtin EC3 Y=0.755 7X+4.571 5 3.69 1.80~7.56 0.988 1 5 Y=1.046 1X+4.924 4 1.18 0.83~1.68 0.988 7 7 Y=1.465 2 X+5.333 8 0.59 0.46~0.76 0.990 5 40%印楝素干粉
40% Azadirachtin
dry powder3 Y=0.947 7 X+4.353 0 4.82 2.46~9.45 0.996 7 5 Y=0.932 1 X+4.616 5 2.58 1.53~4.33 0.990 1 7 Y=0.977 4 X+5.032 7 0.93 0.65~1.32 0.988 3 3龄
3rd instar0.3%印楝素乳油
0.3% Azadira-
chtin EC3 Y=0.871 5 X+4.671 7 2.38 1.40~4.04 0.990 5 5 Y=1.179 9 X+5.057 4 0.89 0.66~1.21 0.989 2 7 Y=1.495 5 X+5.500 3 0.46 0.36~0.60 0.991 1 40%印楝素干粉
40% Azadirachtin
dry powder3 Y=0.859 5 X+4.481 7 4.01 2.06~7.81 0.992 4 5 Y=0.831 5 X+4.600 1 3.03 1.65~5.56 0.993 0 7 Y=1.304 2 X+5.136 4 0.79 0.60~1.03 0.989 1 1) Y:死亡概率,X:浓度对数
1) Y: Mortality probability, X: Logarithm of concentration从表1可以看出,相较于40%印楝素干粉,0.3%印楝素乳油对草地贪夜蛾有更好的毒力,处理后7 d 2龄和3龄幼虫的LC50值为0.59和0.46 mg/L,而40%印楝素干粉处理草地贪夜蛾后7 d,2龄和3龄幼虫的LC50值为0.93和0.79 mg/L。试验中,印楝素对草地贪夜蛾3龄幼虫的毒杀活性要高于2龄幼虫。
2.2 印楝素对草地贪夜蛾幼虫的室内拒食活性
本文为评价使用印楝素对草地贪夜蛾的拒食活性与防效,在室内测定了0.3%印楝素乳油和40%印楝素干粉对草地贪夜蛾幼虫的拒食活性,结果见表2。从表2可以看出,印楝素对草地贪夜蛾3龄幼虫的拒食效果要较好于2龄幼虫;相较于40%印楝素干粉,0.3%印楝素乳油对草地贪夜蛾具有更好的拒食活性,处理后3 d,2龄和3龄幼虫的AFC50值分别为0.30和0.12 mg/L;而40%印楝素干粉处理草地贪夜蛾后3 d,2龄和3龄幼虫的AFC50值分别为0.53和0.30 mg/L。试验观察发现,处理组的幼虫出现逃避叶片、显著减少取食的现象。
表 2 印楝素对草地贪夜蛾2龄和3龄幼虫的拒食活性Table 2. The anti-feedant activities of azadirachtin on the 2nd and 3 rd instar larvae of Spodoptera frugiperda幼虫
Larva处理
Treatmentt/d 毒力回归方程1)
Regression equationAFC50/
(mg·L−1)95%置信区间
95% confidence limit相关系数(r)
Correlation coefficient2龄
2nd instar0.3%印楝素乳油
0.3% Azadira-
chtin EC1 Y=0.839 1X+5.362 5 0.37 0.17~0.82 0.990 6 2 Y=0.699 0X+5.238 7 0.46 0.19~1.10 0.988 0 3 Y=0.643 4X+5.334 6 0.30 0.10~0.88 0.994 2 40%印楝素干粉
40% Azadirachtin
dry powder1 Y=0.744 4X+5.098 9 0.74 0.33~1.62 0.994 9 2 Y=0.708 5X+5.219 6 0.49 0.21~1.15 0.989 8 3 Y=0.667 2X+5.185 5 0.53 0.22~1.29 0.992 0 3龄
3rd instar0.3%印楝素乳油
0.3% Azadira-
chtin EC1 Y=0.751 0X+5.521 4 0.20 0.07~0.60 0.989 3 2 Y=0.846 4X+5.628 2 0.18 0.06~0.51 0.990 0 3 Y=0.671 0X+5.611 7 0.12 0.03~0.54 0.989 7 40%印楝素干粉
40% Azadirachtin
dry powder1 Y=0.815 0X+5.379 8 0.34 0.15~0.79 0.995 3 2 Y=0.738 3X+5.531 5 0.19 0.06~0.59 0.988 6 3 Y=0.784 1X+5.408 9 0.30 0.12~0.74 0.997 0 1) Y:死亡概率,X:浓度对数
1) Y: Mortality probability, X: Logarithm of concentration2.3 印楝素对草地贪夜蛾的田间防效
田间测定了0.3%印楝素乳油对草地贪夜蛾的防治效果,并与10%(φ)氯氰菊酯乳油1 000倍液进行比较,结果见表3。施药后观察,0.3%印楝素乳油对玉米安全,玉米正常生长,无药害产生,对非靶标生物也未见明显的不良影响。从表3可以看出,在印楝素药剂稀释500倍时,施药后7 d,0.3%印楝素乳油对草地贪夜蛾具有良好的防效,可以将虫口密度控制在较低的水平。稀释500倍时,施药后1、3和7 d防效分别达到24.83%、50.34%和75.50%,稀释800倍时,施药后1、3和7 d的防效分别为19.76%、41.51%和66.03%,稀释1 000倍时,施药后1、3和7 d的防效分别为10.90%、32.70%和53.37%。试验发现,0.3%印楝素乳油3个处理浓度之间,稀释倍数越低,即浓度越高,对草地贪夜蛾的防效越佳。施药后草地贪夜蛾对小区玉米再取食率低,叶片的完整程度较为理想;草地贪夜蛾的卵块明显减少,但0.3%印楝素乳油防治草地贪夜蛾的速效性不佳。
表 3 0.3%印楝素乳油对草地贪夜蛾的田间防效1)Table 3. The field control effect of 0.3% azadirachtin EC for Spodoptera frugiperda处理 Treatment 稀释倍数 Dilution times 药后不同时间的防效/% Control effect at different time after treatment 1 d 3 d 7 d 0.3%印楝素乳油 500 24.83±1.46b 50.34±2.92b 74.50±4.38a 0.3% Azadirachtin EC 800 19.76±3.09b 41.51±0.83c 66.03±3.31ab 1 000 10.90±0.99c 32.70±2.97d 53.37±1.71bc 10%氯氰菊酯乳油
10% Cypermethrin EC1 000 57.63±1.61a 66.67±1.84a 44.62±3.06c 1)同列数据后凡是有一个相同字母者,表示差异不显著(P>0.05,Tukey法)
1) The same letters in the same column indicated that the difference was not significant (P>0.05, Tukey method)3. 讨论与结论
本文通过测定印楝素对草地贪夜蛾的毒杀活性、拒食活性以及防治效果,评价了印楝素在草地贪夜蛾防治工作中的应用潜力。试验中发现,相较于40%印楝素干粉,0.3%印楝素乳油对草地贪夜蛾具有更好的毒力。在生产过程中,40%印楝素干粉经除杂净化,会去除一些活性物质,而0.3%印楝素乳油中的活性物质较多,这些活性物质与印楝素发生一定的协同作用,从而造成0.3%印楝素乳油对草地贪夜蛾的毒力作用优于40%印楝素干粉。对比2龄幼虫和3龄幼虫,发现印楝素对3龄幼虫具有更好的毒杀活性和拒食活性。一般情况下,鳞翅目1~2龄害虫是对农药抵抗力最弱的阶段,杀虫剂对鳞翅目2龄害虫的毒力作用要强于3龄幼虫[20]。本研究对草地贪夜蛾取食行为的观察发现,2龄幼虫由于吃不穿叶片,取食后叶片还留下一层膜,取食同等的叶面积,2龄幼虫取食的药量接近3龄幼虫取食药量的一半,因而造成印楝素对草地贪夜蛾3龄幼虫的毒力作用强于2龄幼虫。
抗药性是杀虫剂使用留给害虫的遗传特征[21],草地贪夜蛾作为一种迁飞性害虫,无疑会将抗性基因扩散,从而放大抗药性的问题,抗药性问题会增加农药的使用剂量,进而引发农产品的质量安全和环境安全等问题。生物农药对环境安全,并且由于具有多重作用机制不易引起抗药性,目前已成为防治害虫的重要手段之一[22],虽然试验中印楝素对草地贪夜蛾的防治时效性不佳,但印楝素作为植物杀虫剂的代表,几乎达到了理想农药所要求的全部标准,不仅无明显的脊椎动物毒性或植物药害,还能在环境中迅速降解[23]。试验中发现,印楝素不仅对草地贪夜蛾具有良好的毒杀活性,还具有明显的拒食活性,在田间施药后,草地贪夜蛾对玉米取食率明显降低,叶片较为完整,在印楝素毒杀和拒食双重作用机制下,草地贪夜蛾的危害得到了有效控制。综上所述,在草地贪夜蛾的防治工作中,印楝素具有广阔的应用前景。
-
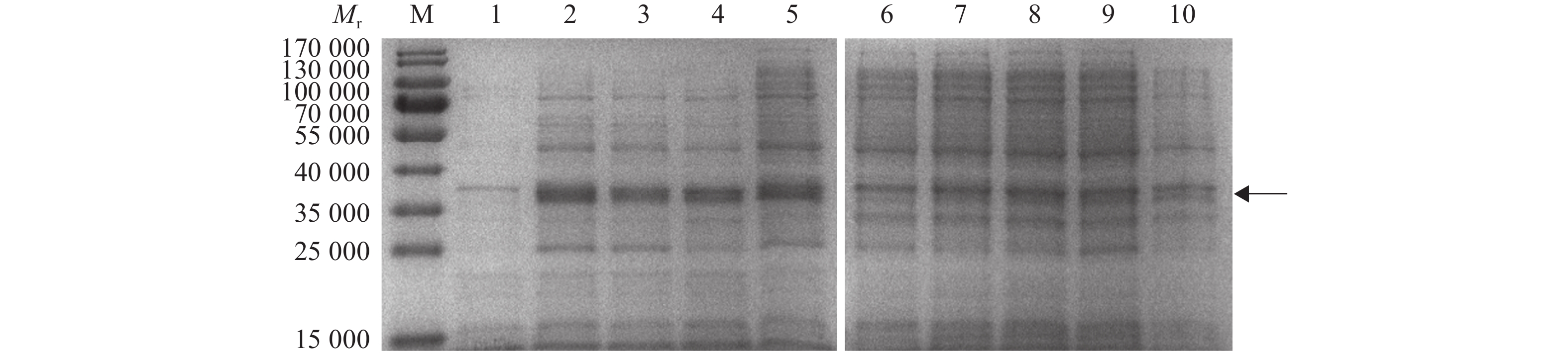
图 11 18、28、37 ℃诱导不同时间结果
各小图中,Mr:相对分子质量;M:蛋白marker;1、3、5、7分别为诱导4、8、12、16 h上清液,2、4、6、8分别为诱导4、8、12、16 h沉淀
Figure 11. Result of different induction time under 18, 28, 37 ℃
In each figure, Mr: Relative molecular mass; M: Protein marker; 1, 3, 5, 7 were induced supernatant, and 2, 4, 6, 8 were induced precipitation respectively for 4, 8, 12, 16 h
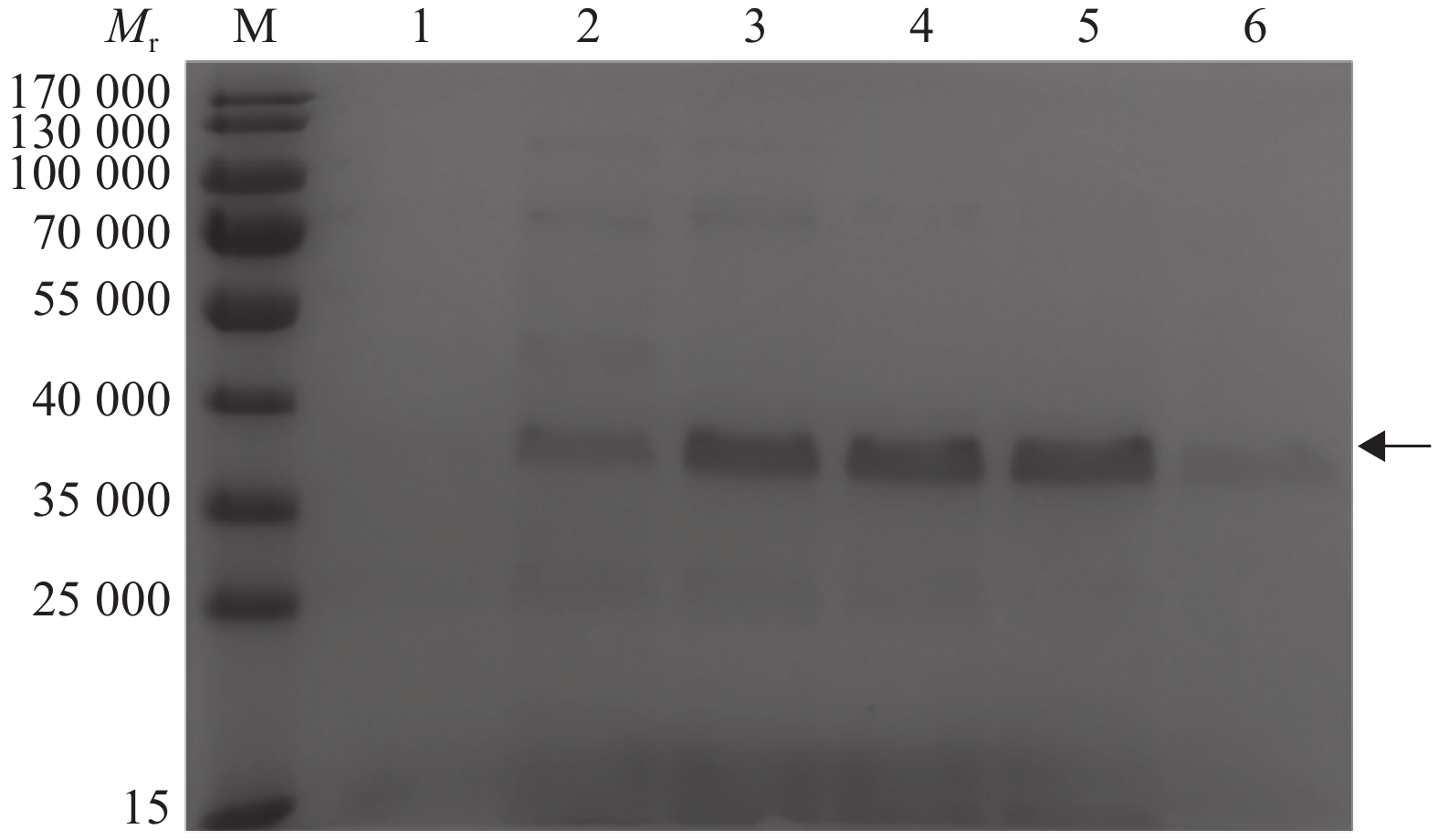
图 12 不同IPTG浓度诱导结果
Mr:相对分子质量;M:蛋白marker;1~5分别为0、0.2、0.5、0.8和1.0 mmol/L IPTG诱导上清液,6~10分别为诱导沉淀
Figure 12. Induction result of different IPTG concentrations
Mr: Relative molecular mass; M: Protein marker; 1−5 were induced supernatant and 6−10 were induced precipitation respectively by 0, 0.2, 0.5, 0.8 and 1.0 mmol/L IPTG
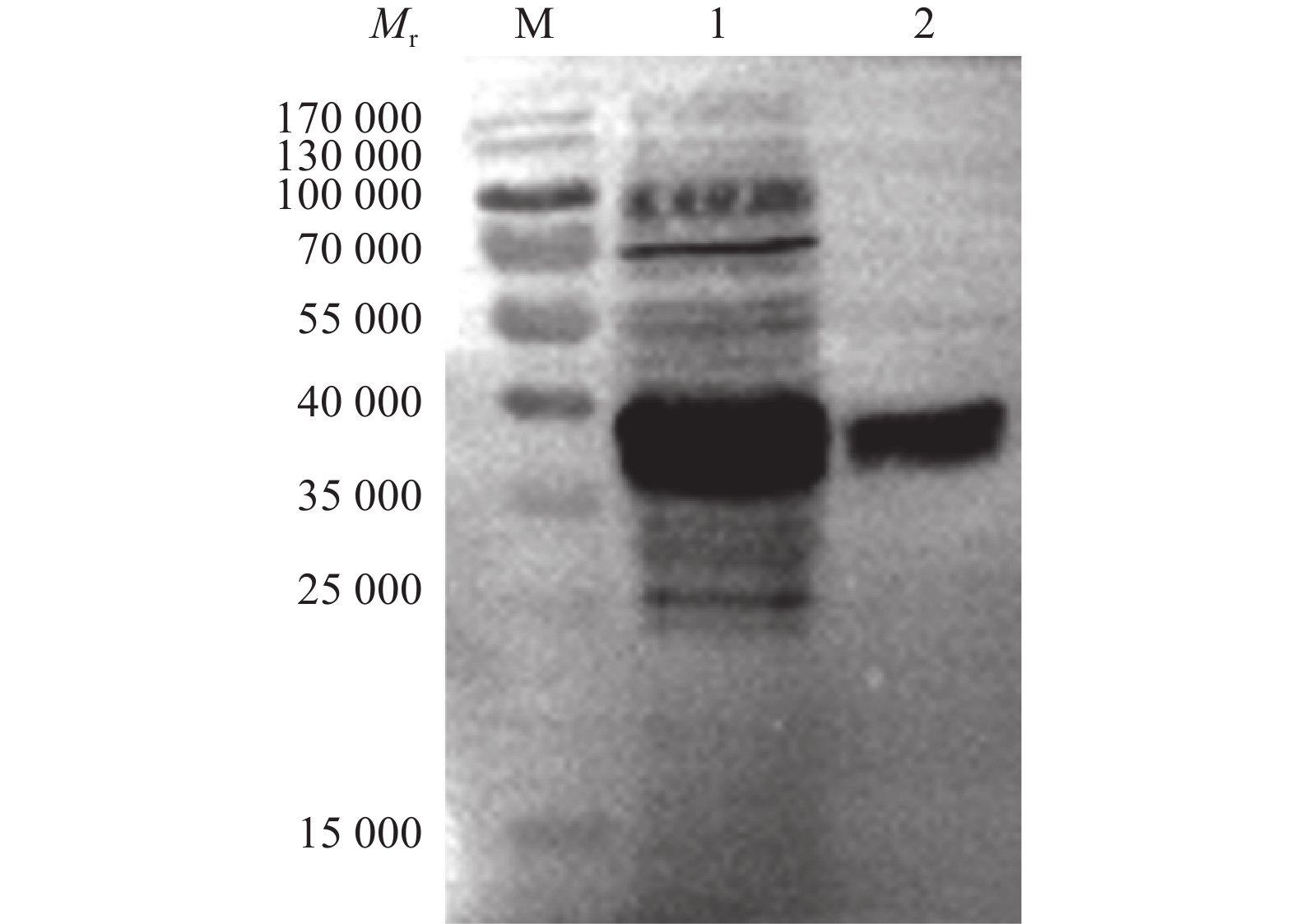
图 13 不同浓度梯度的咪唑洗脱液的SDS-PAGE鉴定
Mr:相对分子质量;M:蛋白marker;1~6分别为20、50、100、150、200和250 mmol/L咪唑洗脱液
Figure 13. Identification of imidazole eluents with different concentration gradients by SDS-PAGE
Mr: Relative molecular mass; M: Protein marker; 1−6 were 20, 50, 100, 150, 200 and 250 mmol/L imidazole eluents, respectively
-
[1] DIONE M, MASEMBE C, AKOL J, et al. The importance of on-farm biosecurity: Sero-prevalence and risk factors of bacterial and viral pathogens in smallholder pig systems in Uganda[J]. Acta Tropica, 2018, 187: 214-221. doi: 10.1016/j.actatropica.2018.06.025
[2] 刘丽. 猪球虫病的流行病学、临床症状、诊断、鉴别及防治[J]. 现代畜牧科技, 2021(2): 121-122. [3] GONG Q L, ZHAO W X, WANG Y C, et al. Prevalence of coccidia in domestic pigs in China between 1980 and 2019: A systematic review and meta-analysis[J]. Parasites & Vectors, 2021, 14(1): 248.
[4] PALMIERI N, SHRESTHA A, RUTTKOWSKI B, et al. The genome of the protozoan parasite Cystoisospora suis and a reverse vaccinology approach to identify vaccine candidates[J]. International Journal for Parasitology, 2017, 47(4): 189-202. doi: 10.1016/j.ijpara.2016.11.007
[5] 杨守深, 孙晓双, 邱云飞, 等. 猪囊等孢球虫孢子化与未孢子化卵囊的差异表达蛋白质组学分析[J]. 畜牧兽医学报, 2020, 51(9): 2216-2226. doi: 10.11843/j.issn.0366-6964.2020.09.019 [6] CRAWFORD J, TONKIN M L, GRUJIC O, et al. Structural characterization of apical membrane antigen 1 (AMA1) from Toxoplasma gondii[J]. Journal of Biological Chemistry, 2010, 285(20): 15644-15652. doi: 10.1074/jbc.M109.092619
[7] TRIGLIA T, HEALER J, CARUANA S R, et al. Apical membrane antigen 1 plays a central role in erythrocyte invasion by Plasmodium species[J]. Molecular Microbiology, 2000, 38(4): 706-718. doi: 10.1046/j.1365-2958.2000.02175.x
[8] HEHL A B, LEKUTIS C, GRIGG M E, et al. Toxoplasma gondii homologue of Plasmodium apical membrane antigen 1 is involved in invasion of host cells[J]. Infection and Immunity, 2000, 68(12): 7078-7086. doi: 10.1128/IAI.68.12.7078-7086.2000
[9] MITAL J, MEISSNER M, SOLDATI D, et al. Conditional expression of Toxoplasma gondii apical membrane antigen-1 (TgAMA1) demonstrates that TgAMA1 plays a critical role in host cell invasion[J]. Molecular Biology of the Cell, 2005, 16(9): 4341-4349. doi: 10.1091/mbc.e05-04-0281
[10] 罗伏兵, 刘晓冬, 梁琳, 等. 巨型艾美耳球虫AMA1蛋白单克隆抗体的制备及其功能鉴定[J]. 中国兽医杂志, 2020, 56(11): 34-36. [11] 王晔, 赵其平, 朱顺海, 等. 柔嫩艾美耳球虫AMA1基因DNA疫苗的免疫保护效果[J]. 中国动物传染病学报, 2019, 27(6): 1-9. [12] NIU Q, LIU Z, YANG J, et al. Molecular cloning, characterization and antigenicity of Babesia sp. BQ1 (Lintan) (Babesia cf. motasi) apical membrane antigen-1 (AMA-1)[J]. Parasitology, 2017, 144(5): 641-649. doi: 10.1017/S0031182016002304
[13] CAZZANIGA G, MORI M, CHIARELLI L R, et al. Natural products against key Mycobacterium tuberculosis enzymatic targets: Emerging opportunities for drug discovery[J]. European Journal of Medicinal Chemistry, 2021, 224: 113732. doi: 10.1016/j.ejmech.2021.113732
[14] WANI W Y, BOYER-GUITTAUT M, DODSON M, et al. Regulation of autophagy by protein post-translational modification[J]. Laboratory Investigation, 2015, 95(1): 14-25. doi: 10.1038/labinvest.2014.131
[15] XIAO J, ZHENG R, BAI X, et al. Preliminary evaluation of the protective effects of recombinant AMA1 and IMP1 against Eimeria stiedae infection in rabbits[J]. Parasites & Vectors, 2022, 15(1): 400.
-
期刊类型引用(5)
1. 李秋洁,王诗瑶,黄政. 基于单线激光雷达的果园车辆地头导航方法. 林业工程学报. 2025(01): 128-135 .  百度学术
百度学术
2. 张日红,陈德照,王振豪,佘梓鹏,王宝娥. 宽行距果蔬种植环境土壤检测机器人设计与试验. 农业机械学报. 2025(02): 217-228 .  百度学术
百度学术
3. 金少宇,宋超,徐子程,邵立奇. 基于激光雷达的果园机器人密度自适应行间导航方法. 南京工程学院学报(自然科学版). 2025(01): 49-55 .  百度学术
百度学术
4. 韩雨晓,李帅,王宁,安娅军,张漫,李寒. 基于3D激光雷达的鸡舍通道中心线检测方法. 农业工程学报. 2024(09): 173-181 .  百度学术
百度学术
5. 吕强,张海涛,王辉,李永强. 基于双目视觉技术的复杂环境下机器人自动导航研究. 机械设计与制造工程. 2023(09): 79-84 .  百度学术
百度学术
其他类型引用(3)




 下载:
下载: