Eukaryotic expression and bioactivity verification of porcine prolactin
-
摘要:目的
催乳素(Prolactin,PRL)具有广泛的生理调节作用,但其多效性机制仍不清楚。为了更好地研究猪PRL的多效性,本研究制备猪源PRL真核重组蛋白并验证其生物活性。
方法利用分子克隆技术将猪PRL基因克隆到慢病毒表达载体pCDH-CMV-MCS-EF1-GFP+Puro中,经慢病毒包装获得携带猪PRL基因的PRL−慢病毒;用浓缩的PRL−慢病毒感染CHO-K1细胞,经嘌呤霉素筛选后,获得能够分泌PRL重组蛋白的阳性细胞系CHO-K1-PRL;利用镍柱亲和层析法对重组蛋白进行纯化并进行LC-MS/MS质谱鉴定,利用HC11细胞体外培养体系验证PRL重组蛋白的生物活性。
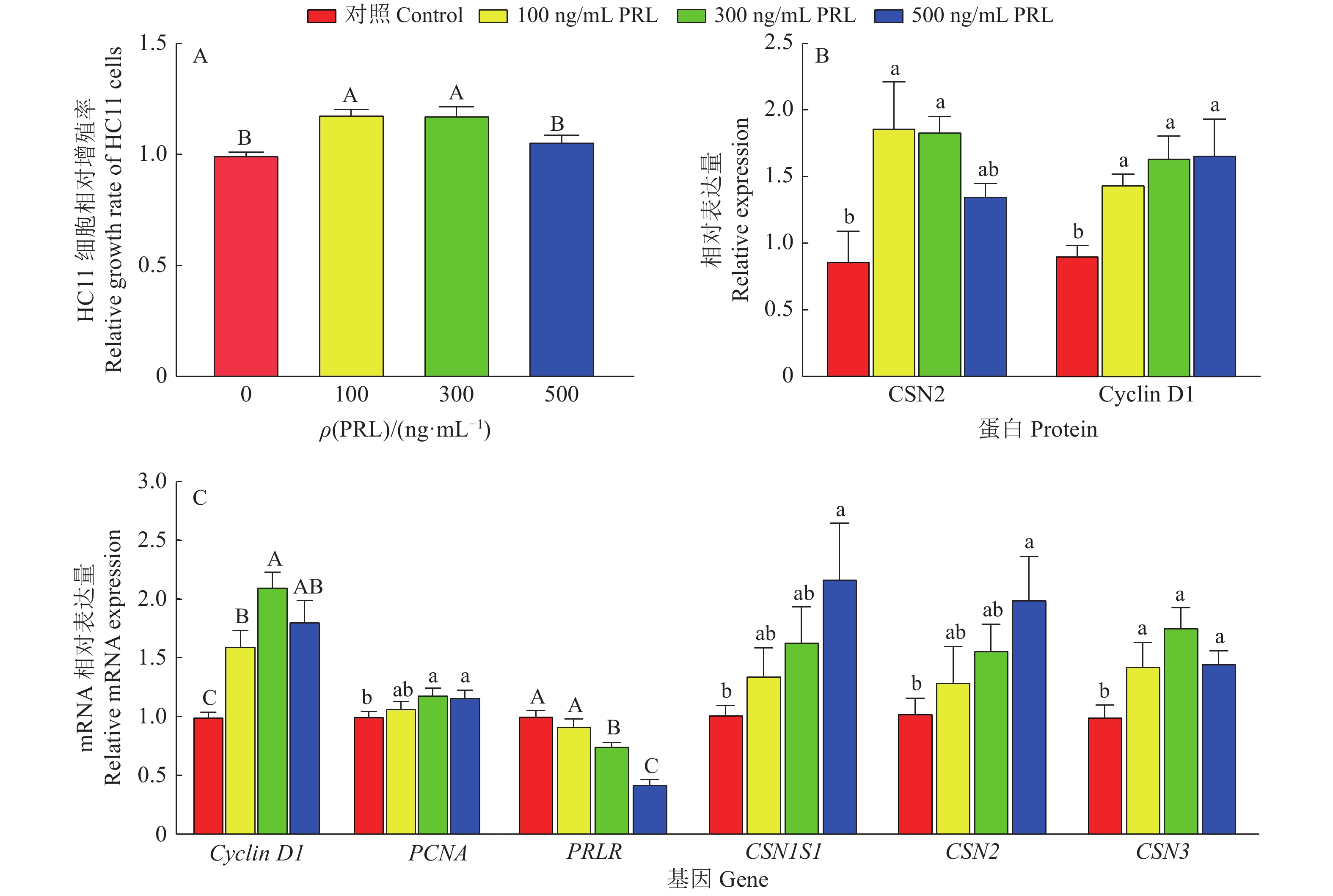
结果成功构建了携带猪PRL基因的pCDH-CMV-6His-PRL-6His-EF1-GFP+Puro慢病毒表达载体;包装及浓缩后的PRL−慢病毒滴度为9.9×108 TU/mL,其感染的CHO-K1细胞经嘌呤霉素筛选后得到阳性细胞系CHO-K1-PRL;从CHO-K1-PRL细胞培养液中成功纯化出重组蛋白,质量浓度为50 μg/mL,LC-MS/MS质谱分析的覆盖率达94%,鉴定为猪PRL重组蛋白;重组PRL具有促进HC11细胞增殖及酪蛋白表达的生物活性。
结论构建的细胞系CHO-K1-PRL可稳定表达具有生物活性的猪重组PRL,为猪PRL功能的研究和生产应用奠定了基础。
Abstract:ObjectiveProlactin (PRL) has a wide range of physiological regulatory effects, but its pleiotropic mechanism is still unclear. In order to investigate the pleiotropy of porcine PRL, we obtain porcine PRL eukaryotic recombinant protein and verify its biological activity.
MethodThe porcine PRL gene was cloned into the lentiviral vector pCDH-CMV-MCS-EF1-GFP+Puro by molecular cloning technology, and the PRL-lentivirus carrying porcine PRL gene was obtained by lentivirus packaging. CHO-K1 cells were infected by the concentrated PRL-lentivirus solution, and the positive cell line named CHO-K1-PRL, which could secrete recombinant PRL protein, was obtained after purinomycin screening. The recombinant protein was purified by nickel column affinity chromatography, and identified by LC-MS/MS mass spectrometry. The biological activity of recombinant PRL was verified by adding recombinant PRL into HC11 cell culture system in vitro.
ResultThe recombinant expression vector pCDH-CMV-6His-PRL-6His-EF1-GFP+Puro carrying porcine PRL gene was successfully constructed. The titer of PRL-lentivirus after concentrating was 9.9×108 TU/mL, and the positive cell line CHO-K1-PRL was obtained after puromycin screening. The recombinant protein with the mass concentration of 50 μg/mL was successfully purified from the supernatant of CHO-K1-PRL cells. The recombinant porcine PRL protein was identified as porcine PRL by LC-MS/MS mass spectrometry, and the coverage rate of recombinant PRL was 94%. The recombinant PRL had the biological activity of promoting the proliferation and casein expression of HC11 cells.
ConclusionThe cell line CHO-K1-PRL constructed in this study can stably express porcine recombinant PRL with biological activity, which lays a foundation for the functional research, production and application of porcine PRL.
-
Keywords:
- Porcine /
- Prolactin /
- CHO-K1 cell /
- Eukaryotic expression /
- Lentiviral vector /
- Recombinant protein
-
激素在哺乳动物生长发育过程中具有不可替代的调控作用,其中直接调控乳腺发育和泌乳的激素主要有雌激素(Estrogen, E)、孕酮(Progesterone,P4)、催乳素(Prolactin, PRL)、生长激素(Growth hormone, GH)、胰岛素(Insulin, INS)和氢化可的松(Hydrocortisone, HC)等[1-2],它们与转化生长因子β1(Transforming growth factor β1,TGF-β1)、信号转导和转录激活因子5(Signal transducer and activator of transcription 5,STAT5) 和胰岛素样生长因子1(Insulin-like growth factor I,IGF-I)等[3]细胞因子相互作用而形成乳腺发育和泌乳的调控网络。目前运用中药组方对人和小鼠等动物催乳或回乳的研究已有报道[4-5],均显示与泌乳激素及相关因子呈一定的相关性[6-7],而奶牛经过泌乳期,机体消耗严重,需要经过回乳期进入干乳期,进而恢复自身体质及更新乳腺组织,为下一个泌乳期做充分准备,但传统回乳方式效果差,易产生乳房炎、流产和抗生素残留等副作用[8],因此,回乳中药组方在奶牛回乳期的运用有着较广阔的研究前景[9]。探究回乳中药组方对回乳期奶牛血清泌乳相关激素、因子、日产奶量及回乳时间的影响,有助于研究开发奶牛乳房保健产品、改进奶牛回乳方法、降低回乳期奶牛乳房炎发生率。本研究旨在探究中药组方“回乳康”对回乳期奶牛血清E、P4和TGF-β1含量的影响,为进一步研究中药组方对回乳期奶牛相关泌乳激素的调控机理奠定基础,并为更安全有效的回乳技术措施提供理论依据。
1. 材料与方法
1.1 试验动物
选择四川省某规模化奶牛场半封闭统一舍饲,体质量(582±41) kg、2~4胎中国荷斯坦奶牛150头。从中选取体况良好,乳房、乳汁均正常,即将进入回乳期,日产奶量为(15.42±0.71) kg的妊娠后期健康奶牛80头。随机分为4组(包括1个对照组和3个中药组),每组20头。各组均采用逐渐干奶法回乳,回乳当天记为第0天,对照组不饲喂中药,从第1天开始,3个中药组每日8:00分别饲喂中药组方“回乳康”400、500和600 g,直至停奶,停止饲喂中药。
1.2 回乳方式
本试验采用逐渐干奶法回乳[10-11],方法为:停喂多汁饲料,减少精料喂量,以青干草为主,控制饮水,适当加强运动。在回乳第1天,挤奶次数由3次改为2次,第2天改为1次,逐渐减少挤奶频率,当奶牛日产奶量为3~5 kg时,停止挤奶。
1.3 试验药物和试剂
中药组方“回乳康”由四川农业大学动物医学院奶牛疾病研究中心研发。由麦芽、朴硝、升麻、柴胡、香附、薏仁、蚕蜕、白术、黄芩、知母、苏梗、芡实、五味子、蒲公英和甘草等组成。
牛E、P4和TGF-β1双抗体夹心ELISA试剂盒,均由美国RD公司提供。
1.4 试验方法
1.4.1 血清收集
试验奶牛回乳开始当天记为第0天,依次采集第0、1、3、5、7、9和11天尾静脉血10 mL,置于未加抗凝剂的离心管中,室温下静置1 h,1 800 r·min-1离心10 min,转移上层血清于EP管中,-20 ℃冻存,待检。
1.4.2 ELISA检测
采用双抗体夹心ELISA测定牛E、P4和TGF-β1的含量,步骤按照说明书进行。
1.5 统计分析
利用SPSS 9.0软件进行统计学分析,K-S检验计量资料是否服从正态分布,数据结果以平均数±标准差表示,2组间采用独立样本t检验,多组间比较采用LSD单因素方差分析,相关性分析采用双变量Pearson相关分析。
2. 结果与分析
2.1 “回乳康”对回乳期奶牛日产奶量的影响
如表 1所示,各组奶牛日产奶量在回乳期均呈下降趋势,回乳期分别为11、7、5和5 d,回乳第0天,各组间奶牛日产奶量差异均不显著(P>0.05);第1天,400 g中药组奶牛日产奶量极显著高于600 g中药组(P<0.01);第3~5天,对照组、400 g中药组奶牛日产奶量均极显著高于600 g中药组,且对照组奶牛日产奶量极显著高于400 g中药组;第7天,对照组奶牛日产奶量极显著高于400 g中药组;500、600 g中药组奶牛日产奶量在整个回乳期差异均不显著。结果表明中药组方“回乳康”具有较好回乳效果。
表 1 中药组方“回乳康”对回乳期奶牛日产奶量的影响1)Table 1. The effects of Chinese herbal formula "Huirukang" on milk production of dairy cows during the milk withdrawal periodkg 时间 对照组 400 g中药组 500 g中药组 600 g中药组 第0天 15.43±0.60a 15.22±0.71a 15.27±0.72a 15.76±0.84a 第1天 15.30±0.92abAB 15.91±0.57aA 15.22±0.75abAB 14.45±0.68bB 第3天 13.33±0.42aA 10.23±1.16bB 9.38±0.92bcBC 8.47±0.88cC 第5天 10.44±0.74aA 7.45±0.94bB 4.85±2.00cC 3.94±0.51cC 第7天 8.08±0.74aA 4.31±0.68bB 第9天 6.73±1.06 第11天 4.38±0.73 1) 同行数据后凡具有一个相同小写、大写字母者,表示不同处理间差异未达到0.05、0.01的显著水平(LSD法,n=20)。 2.2 “回乳康”对回乳期奶牛血清E含量的影响
如表 2所示,各组奶牛血清E含量在回乳期均呈下降趋势。回乳第0至1天,各组奶牛血清E含量差异均不显著;第3—11天,对照组奶牛血清E含量极显著高于其他3组,其中第3—7天,400 g中药组奶牛血清E含量极显著高于500、600 g中药组;500、600 g中药组奶牛血清E含量在整个回乳期差异均不显著。结果表明中药组方“回乳康”可促使回乳期奶牛血清E含量降低。
表 2 中药组方“回乳康”对回乳期奶牛血清E含量的影响1)Table 2. The effects of Chinese herbal formula "Huirukang" on serum estrogen levels of dairy cows during the milk withdrawal periodpg·mL-1 时间 对照组 400 g中药组 500 g中药组 600 g中药组 第0天 1162.76±35.37a 1149.52±32.24a 1148.89±40.66a 1153.30±57.97a 第1天 1176.33±44.31a 1179.19±30.32a 1184.86±27.10a 1149.52±22.87a 第3天 985.95±24.13aA 896.42±32.24bB 839.64±22.48cC 851.11±11.73cC 第5天 967.82±34.40aA 838.03±23.07bB 753.12±11.92cC 749.18±14.14cC 第7天 891.61±15.75aA 810.40±19.86bB 748.48±7.49cC 757.06±12.41cC 第9天 859.05±38.67aA 746.46±12.69bB 753.11±12.10bB 748.60±17.11bB 第11天 843.85±16.12aA 744.63±13.64bB 747.91±16.64bB 756.69±17.11bB 1) 同行数据后凡具有一个相同小写、大写字母者,表示不同处理间差异未达到0.05、0.01的显著水平(LSD法,n=20)。 2.3 “回乳康”对回乳期奶牛血清P4含量的影响
如表 3所示,对照组奶牛血清P4含量在回乳期无明显变化,400、500、600 g中药组均呈先上升后下降的趋势。回乳第0—1天,各组奶牛血清P4含量差异均不显著;从第3天开始,对照组奶牛血清P4含量较其他组低,于第3天显著低于500、600 g中药组,第5—11天则显著低于其他3组。500、600 g中药组P4含量在整个回乳期差异均不显著。结果表明中药组方“回乳康”可促使回乳期奶牛血清P4含量上升。
表 3 中药组方“回乳康”对回乳期奶牛血清P4含量的影响1)Table 3. The effects of Chinese herbal formula "Huirukang" on serum progesterone levels of dairy cows during the milk with-drawal periodng·mL-1 时间 对照组 400 g中药组 500 g中药组 600 g中药组 第0天 14.96±1.54a 14.71±1.18a 15.62±2.73a 15.49±2.09a 第1天 14.51±1.09a 14.83±1.47a 15.09±1.43a 15.99±2.59a 第3天 15.48±1.05aA 16.64±1.09aA 18.46±1.46bB 19.26±0.79bB 第5天 14.69±1.51aA 18.92±0.92bB 24.31±2.55cC 24.70±1.06cC 第7天 15.87±2.12aA 22.87±1.72bB 20.27±0.83bB 22.97±1.82bB 第9天 14.98±1.42aA 21.78±1.92bB 18.53±1.13bB 20.22±1.49bB 第11天 15.31±1.82aA 18.26±1.31bB 19.00±1.61bB 19.04±1.12bB 1) 同行数据后凡具有一个相同小写、大写字母者,表示不同处理间差异未达到0.05、0.01的显著水平(LSD法,n=20)。 2.4 “回乳康”对回乳期奶牛血清TGF-β1含量的影响
如表 4所示,各组奶牛血清TGF-β1含量在回乳期均呈上升趋势,并在回乳期后下降。回乳第0—3天,各组间TGF-β1含量差异均不显著;第7天,对照组、400 g中药组TGF-β1含量极显著高于500、600 g中药组;第9—11天,400 g中药组TGF-β1含量极显著高于其他3组;500、600 g中药组TGF-β1含量在整个回乳期差异均不显著。结果表明中药组方“回乳康”可促使回乳期奶牛血清TGF-β1含量上升。
表 4 中药组方“回乳康”对回乳期奶牛血清TGF-β1含量的影响1)Table 4. The effects of Chinese herbal formula "Huirukang" on serum TGF-β1 levels of dairy cows during the milk with-drawal periodng·mL-1 时间 对照组 400 g中药组 500 g中药组 600 g中药组 第0天 184.20±12.38a 185.78±5.93a 186.20±6.33a 190.33±14.98a 第1天 183.72±9.68a 179.12±11.40a 180.04±8.85a 188.41±10.13a 第3天 236.38±14.34a 239.94±15.58a 234.52±12.07a 232.35±14.10a 第5天 231.87±10.44abA 227.95±11.19aA 233.44±10.79abA 240.51±10.17bA 第7天 243.56±23.85aA 240.11±9.18aA 192.74±24.77bB 187.06±7.89bB 第9天 229.95±15.66abA 199.79±15.72aB 186.57±11.19abB 181.95±7.39bB 第11天 237.68±15.00aA 187.60±6.55bB 186.64±11.83bB 186.42±5.41bB 1) 同行数据后凡具有一个相同小写、大写字母者,表示不同处理间差异未达到0.05、0.01的显著水平(LSD法,n=20)。 2.5 回乳期奶牛血清E、P4、TGF-β1含量与日产奶量的相关性
如表 5所示,在整个回乳期,奶牛血清E、TGF-β1含量和日产奶量两两间均呈极显著相关,血清P4含量与E、TGF-β1含量、日产奶量相关性不显著。
表 5 回乳期奶牛血清PRL、P4、TGF-β1含量与日产奶量的相关性1)Table 5. Correlation between serum estrogen, progesterone and TGF-β1 levels and daily milk yield during the milk with-drawal period指标 日产奶量 E含量 P4含量 TGF-β1含量 r P r P r P r P 日产奶量 0.908** < 0.001 -0.133 0.361 0.657** < 0.001 E含量 -0.155 0.287 -0.767** < 0.001 P4含量 0.244 0.091 TGF-β1含量 1) **代表相关性达到0.01的显著水平。 3. 讨论与结论
3.1 “回乳康”对回乳期奶牛日产奶量的影响
目前,口服麦芽,外敷芒硝等方式促进哺乳期妇女回乳的研究已报道较多[12-13]。叶琳[14]使用麦芽、蒲公英和神曲等使妇女乳汁减少至无乳,王雄[4]使用“回乳抑增一号”(麦芽、牡蛎、浙川贝等)使妇女溢乳改善,郝振华等[5]使用麦芽治疗妇女产后溢乳有明显疗效。现有回乳中药组方中多有麦芽,且已人工合成麦角衍生物——卡麦角林,其明显抑制PRL生成,控制泌乳的进行[15]。然而,中药组方运用于奶牛回乳鲜见报道,本试验结果显示,中药组方“回乳康”具有较好回乳效果,缩短了奶牛回乳期,且有效减少了日产奶量,推测其机理可能是“回乳康”中炒麦芽消食、回乳[4, 13];升麻、柴胡、香附、白术疏肝理气;黄芩、知母、苏梗安胎;五味子、蒲公英固精敛阴,清热解毒,统筹全局,断其生化之源,使肝气调达四运,有效抑制乳汁分泌而回乳。500、600 g中药组奶牛日产奶量在整个回乳期差异均不显著,表明“回乳康”剂量达到500 g·d-1时,效果即可达到最佳。
3.2 “回乳康”对回乳期奶牛血清E含量的影响
E是卵巢和胎盘合成和分泌的一种类固醇激素,是乳腺发育、泌乳启动与维持必不可少的激素之一[16]。乳腺发育早期,E和各类生长因子协同调控导管上皮末端终芽增生,乳腺发育中、后期,E促进乳腺导管生长,诱导P4受体表达,促进乳腺发育,提高乳腺上皮组织PRL水平,并与E受体(Estrogen receptor,ER)结合直接发挥促乳功能,提高泌乳量[17]。同时,E可诱导GH活性,影响INS敏感性,间接调控乳腺泌乳[18]。郝振荣等[19]和Cools等[20]均发现大豆异黄酮(植物雌激素)可显著提高奶牛泌乳量,说明了血清E含量与泌乳量呈正相关。然而,Berryhill等[21]研究表明E可促进产后妇女回乳。本研究结果显示,在奶牛回乳过程中,随泌乳量逐渐下降,血清E含量呈阶梯式下降趋势,结果与郝振荣等[19]和Cools等[20]对E与泌乳量呈正相关的研究报道一致,可能是由于奶牛回乳期泌乳量下降,PRL降低,使得乳腺ER表达减少[22],反馈调节E降低。
李萍萍等[23]利用柴胡、丹皮、紫草等组成的中药组方降低了小鼠血清E含量,Tiosano等[24]研究发现18种中药复合物可改变儿童E含量及活性,王丹等[25]利用“参芪解郁颗粒”增加了妇女产后E含量,王小云等[26]利用熟地、白术、泽泻等组成的中药组方显著降低妇女E表达。本研究结果显示,中药组方“回乳康”使回乳期奶牛血清E含量明显降低每天饲喂500 g即可达到最佳效果,且中药组E含量在回乳期后无明显变化,可能是由于奶牛停止泌乳引起。
3.3 “回乳康”对回乳期奶牛血清P4含量的影响
P4是介于内分泌和免疫系统交互作用的重要因子,具有促进乳腺小叶及腺泡发育,维持妊娠等作用[27]。陈建晖等[28]研究表明,在E刺激乳腺导管发育的基础上,P4能使乳腺发育更充分,黄利等[29]也指出P4可明显刺激豚鼠乳腺增生,王瑞琼等[30]发现乳腺生长不良孕鼠乳腺组织P4及其受体表达量较低。虽然P4可刺激乳腺发育,但不会刺激乳腺泌乳[31],夏成等[32]指出奶牛泌乳量与P4含量无明显相关性。本研究结果显示,在奶牛回乳过程中,对照组P4含量在回乳期差异不显著,可能是由于P4主要是妊娠黄体产生,其在奶牛怀孕后期主要功能是抑制子宫肌蠕动,以维持胎犊宫内生长,而对回乳期奶牛泌乳无明显影响。
王丹等[25]利用“参芪解郁颗粒”显著降低妇女血清P4含量,张剑锋等[33]使用黄芩、苏梗、白术等组成的中药组方提升了怀孕妇女P4表达量,保胎效果良好。本研究使用的中药组方“回乳康”能够极显著提高回乳期奶牛血清P4含量,500 g和600 g的“回乳康”饲喂量对奶牛回乳期血清P4含量影响一致,在回乳期结束后,P4含量有一定程度的下降,但各中药组仍极显著高于对照组,表明奶牛回乳期饲喂中药组方“回乳康”能够提高其血清P4含量,且以每天500 g为佳,可能是中药组方“回乳康”中黄芩、苏梗、白术等中药提高了妊娠奶牛血清P4含量,P4含量的升高使子宫内膜和子宫肌松弛,从而保证胎犊宫内正常生长发育。
3.4 “回乳康”对回乳期奶牛血清TGF-β1含量的影响
TGF-β1是一种多效细胞因子,可影响上皮细胞增殖、凋亡并维持细胞外基质稳态,对乳腺形态发生及分泌功能有重要作用。已有研究表明,TGF-β1可诱导乳腺上皮干细胞群衰老,抑制乳腺发育,并与乳腺泌乳的终止信号密切相关[34]。另外,TGF-β1可减少乳腺上皮细胞中由PRL诱导的β-酪蛋白mRNA和蛋白表达水平,且对蛋白表达水平的抑制更明显[35]。说明TGF-β1在动物乳腺退化过程中对抑制细胞生长起重要调控作用[36],Vries等[37]指出TGF-β1含量表达在奶牛回乳后1周内达到最高,本研究发现,在奶牛回乳过程中,随泌乳量逐渐下降,血清TGF-β1含量呈上升趋势,与上述研究结果趋势一致,可能是由于回乳期奶牛机体缺少挤奶刺激,促使乳腺表达TGF-β1,减少β-酪蛋白mRNA和蛋白表达水平,降低奶牛泌乳量。
张臻等[38]使用黄芩、太子参等组成的中药组方提升了大鼠TGF-β1表达量,葛明晓等[39]利用熟地、山药和白术等组成的中药组方增加了妇女血清TGF-β1含量。本研究使用的中药组方“回乳康”可使奶牛快速回乳,回乳期结束后,各中药组TGF-β1含量均有一定程度的下降,缩短了TGF-β1高含量水平及回乳期持续时间,从而降低对细胞增殖的抑制作用,间接促进胎犊生长发育。而对照组变化不明显,可能是由于对照组奶牛回乳持续时间较长,致使TGF-β1还未达到降低期。
3.5 回乳期奶牛血清E、P4、TGF-β1含量和日产奶量的相关性
在奶牛乳腺生长发育过程中,E可促进乳腺导管上皮细胞和小叶周围结缔组织生长,诱导P4受体表达,促进P4与E协同刺激乳腺上皮细胞DNA合成和腺泡发育[27]。怀孕后期奶牛E和P4含量均升高,乳腺组织发育迅速,但血液中P4含量过高,抑制了泌乳作用,从而使乳腺虽具备泌乳能力而不泌乳[31],分娩后,血液中P4浓度降低,PRL才正常发挥泌乳功能。有研究指出,在乳腺退化过程中,TGF-β1在转录水平及蛋白水平上均有所增加[40],抑制ER表达,诱导乳腺导管快速退化[41]。本研究表明,在奶牛回乳期,E是奶牛回乳的负调控激素,而TGF-β1是奶牛回乳的正调控因子。然而回乳期奶牛血清P4含量与血清E、TGF-β1含量及日产奶量相关性均不显著,可能是由于P4只促进青春期奶牛乳腺发育,而对泌乳期和回乳期奶牛乳腺无明显影响,此时,P4主要是由妊娠黄体分泌,维持妊娠。
-
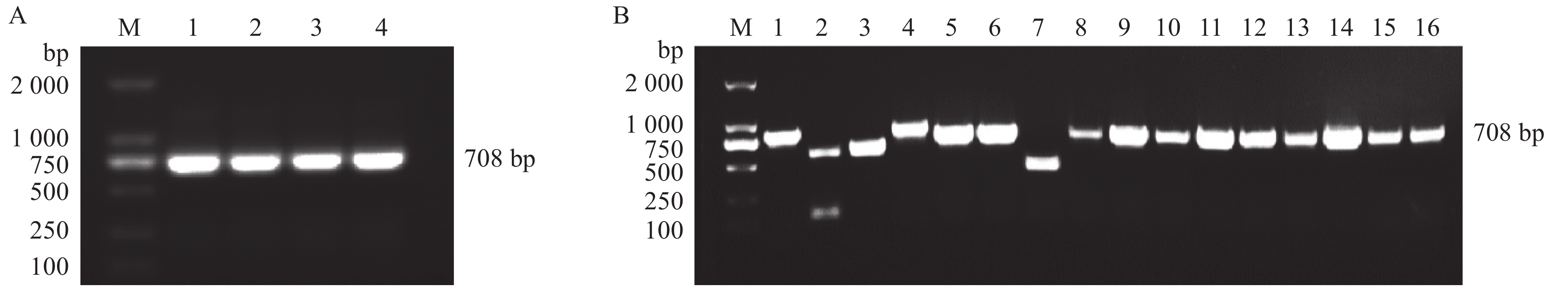
图 1 pMD18-T-PRL克隆载体的构建与PCR鉴定
A:PRL基因的PCR产物,M为DL 2000 DNA Marker,1~4以母猪垂体组织cDNA为模板;B:pMD18-T-PRL菌液PCR产物,M为DL 2000 DNA Marker,1~16分别以挑选的不同pMD18-T-PRL菌落的菌液为模板
Figure 1. Construction and PCR identification of pMD18-T-PRL cloning plasmid
A: PCR products of PRL gene, M is DL 2000 DNA Marker, 1−4 use sow pituitary tissue cDNA as template; B: PCR products of pMD18-T-PRL colonies, M is DL 2000 DNA Marker, 1−16 respectively use different pMD18-T-PRL colonies as template
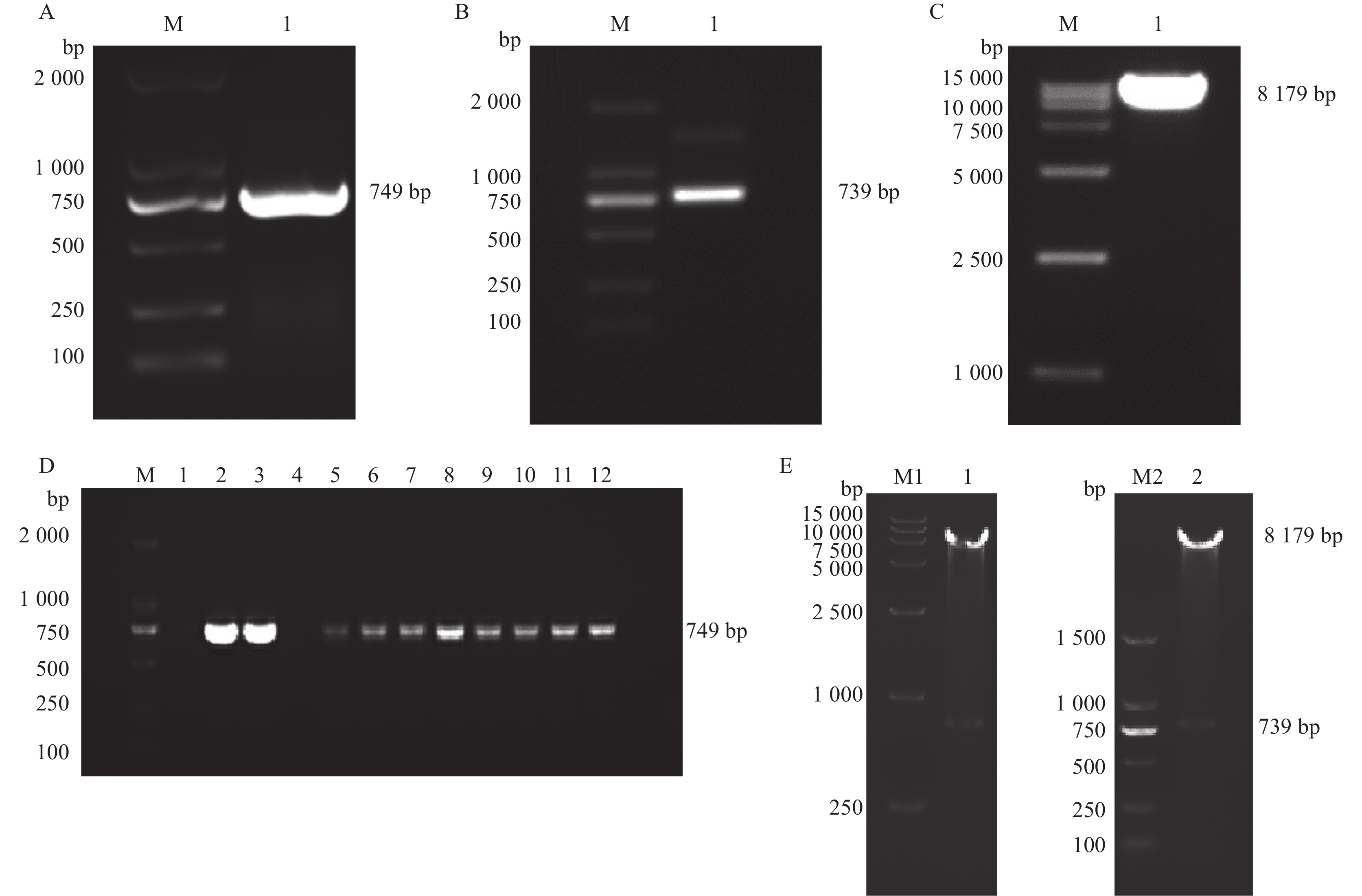
图 2 pCDH-CMV-6His-PRL-6His-EF1-GFP+Puro慢病毒表达载体的构建与PCR鉴定
A:EcoR I-6His-PRL-6His-BamH I序列片段PCR产物,M为DL 2000 DNA Marker,1为EcoR I-6His-PRL-6His-BamH I序列片段;B:EcoR I-6His-PRL-6His-BamH I序列片段双酶切产物,M为DL 2000 DNA Marker,1为6His-PRL-6His片段;C:pCDH-CMV-MCS-EF1-GFP+Puro质粒双酶切产物,M为DL 15000 DNA Marker,1为pCDH-CMV-MCS-EF1-GFP+Puro线性片段;D:pCDH-CMV-6His-PRL-6His-EF1-GFP+Puro菌液PCR产物,M为DL 2000 DNA Marker,1~12分别以挑选的不同单菌落的菌液为模板;E:重组质粒pCDH-CMV-6His-PRL-6His-EF1-GFP+Puro的双酶切结果,M1为DL 15000 DNA Marker,M2为DL 2000 DNA Marker,1和2为重组质粒双酶切片段
Figure 2. Construction and PCR identification of pCDH-CMV-6His-PRL-6His-EF1-GFP+Puro lentivirus expression plasmid
A: PCR product of EcoR I-6His-PRL-6His-BamH I sequence fragment, M is DL 2000 DNA Marker, 1 is EcoR I-6His-PRL-6His-BamH I sequence fragment; B: Double digested product of EcoR I-6His-PRL-6His-BamH I sequence fragment, M is DL 2000 DNA Marker, 1 is 6His-PRL-6His sequence fragment; C: Double digested product of pCDH-CMV-MCS-EF1-GFP+Puro plasmid, M is DL 15000 DNA Marker, 1 is pCDH-CMV-MCS-EF1-GFP+Puro plasmid linear fragment; D: PCR products of pCDH-CMV-6His-PRL-6His-EF1-GFP+Puro colonies, M is DL 2000 DNA Marker, 1−12 respectively use selected bacterial solutions from different single colonies as templates; E: Double digested product of recombinant vector pCDH-CMV-MCS-EF1-GFP+Puro, M1 is DL 15000 DNA Marker, M2 is DL 2000 DNA Marker, 1 and 2 are double digested products of recombinant vector
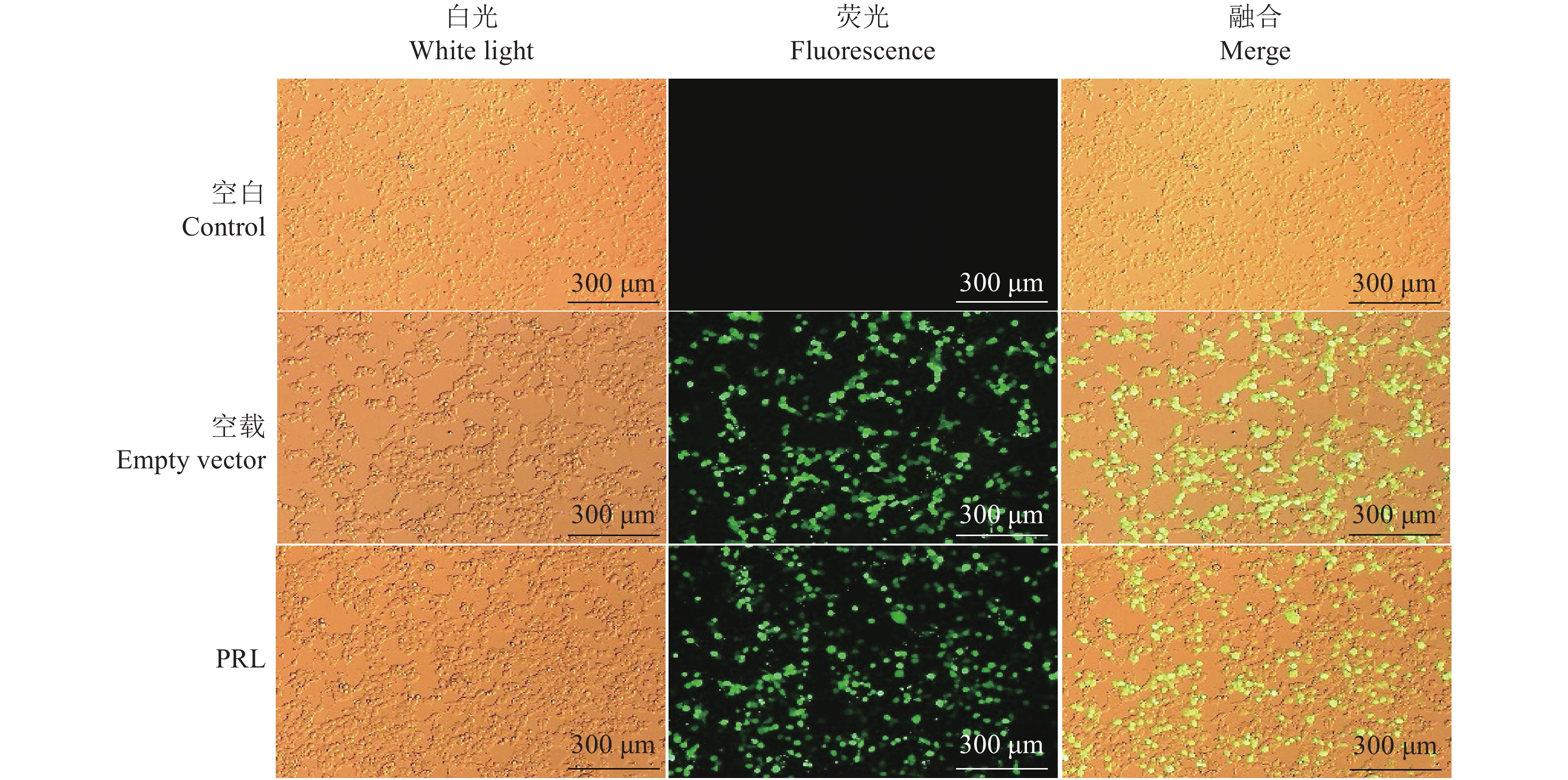
图 3 慢病毒载体转染293T细胞24 h荧光表达情况
空白:未做处理的293T细胞;空载:转染空慢病毒载体的293T细胞;PRL:转染pCDH-CMV-6His-PRL-6His-EF1-GFP+Puro慢病毒表达载体的293T细胞
Figure 3. Fluorescent expression of 293T cells transfected with lentivirus plasmid for 24 h
Control: Untreated 293T cells; Empty vector: 293T cells transfected with empty lentiviral vector; PRL: 293T cells transfected with pCDH-CMV-6His-PRL-6His-EF1-GFP+Puro lentiviral vector
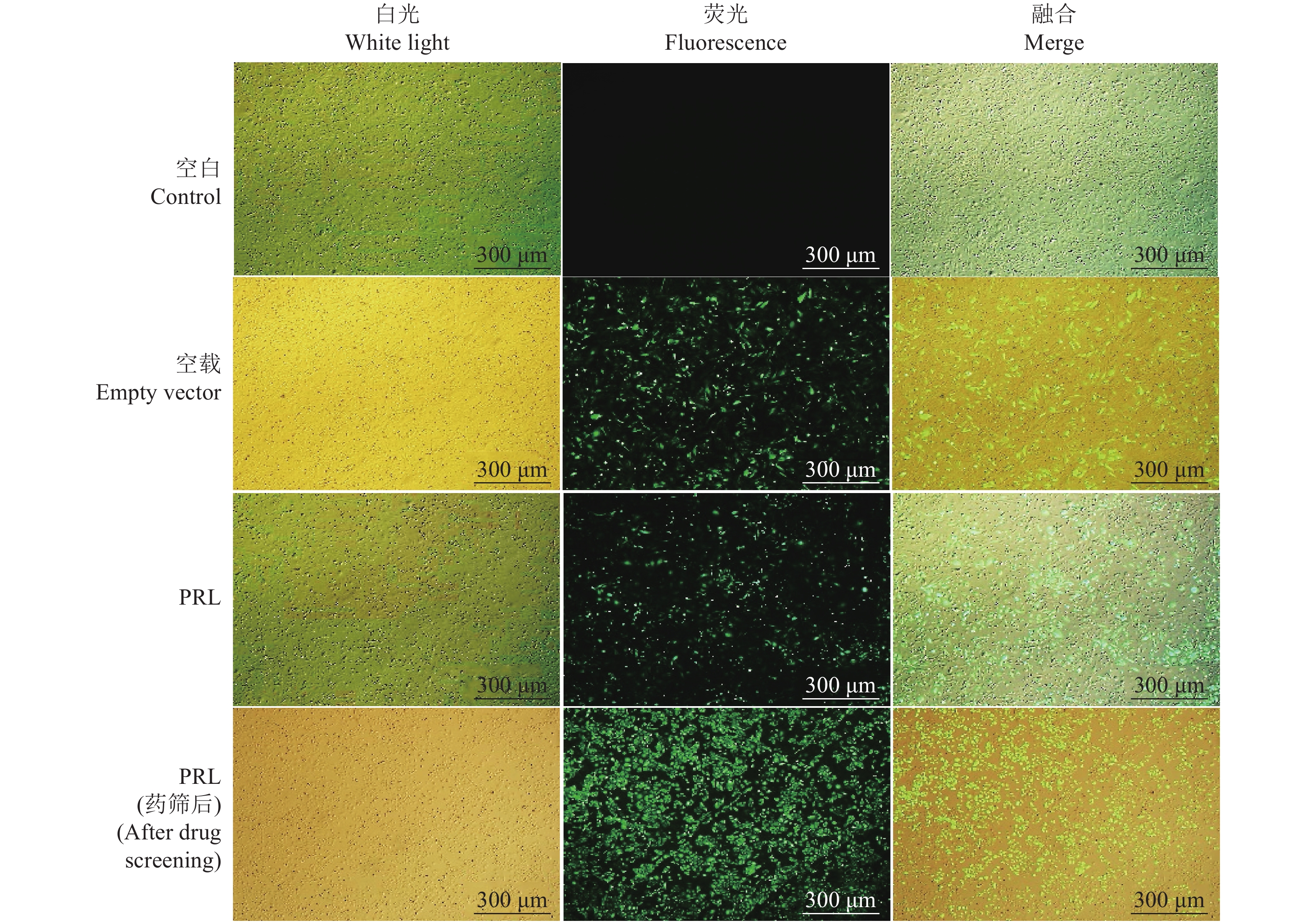
图 4 慢病毒浓缩液感染CHO-K1细胞72 h及药筛7 d后荧光表达情况
空白:未做处理的CHO-K1细胞;空载:感染空载体慢病毒液的CHO-K1细胞;PRL:感染PRL−慢病毒液的CHO-K1细胞;PRL(药筛后):感染PRL−慢病毒液的CHO-K1细胞经嘌呤霉素药物筛选7 d后
Figure 4. Fluorescence expression of CHO-K1 cells infected with concentrated lentivirus solution for 72 h and seven days after drug screening
Control: Untreated CHO-K1 cells; Empty vector: CHO-K1 cells infected by the concentrated empty lentivirus solution; PRL: CHO-K1 cells infected by the concentrated PRL-lentivirus solution; PRL (after drug screening): CHO-K1 cells infected with PRL-lentivirus solution and then screened by purinomycin for seven days
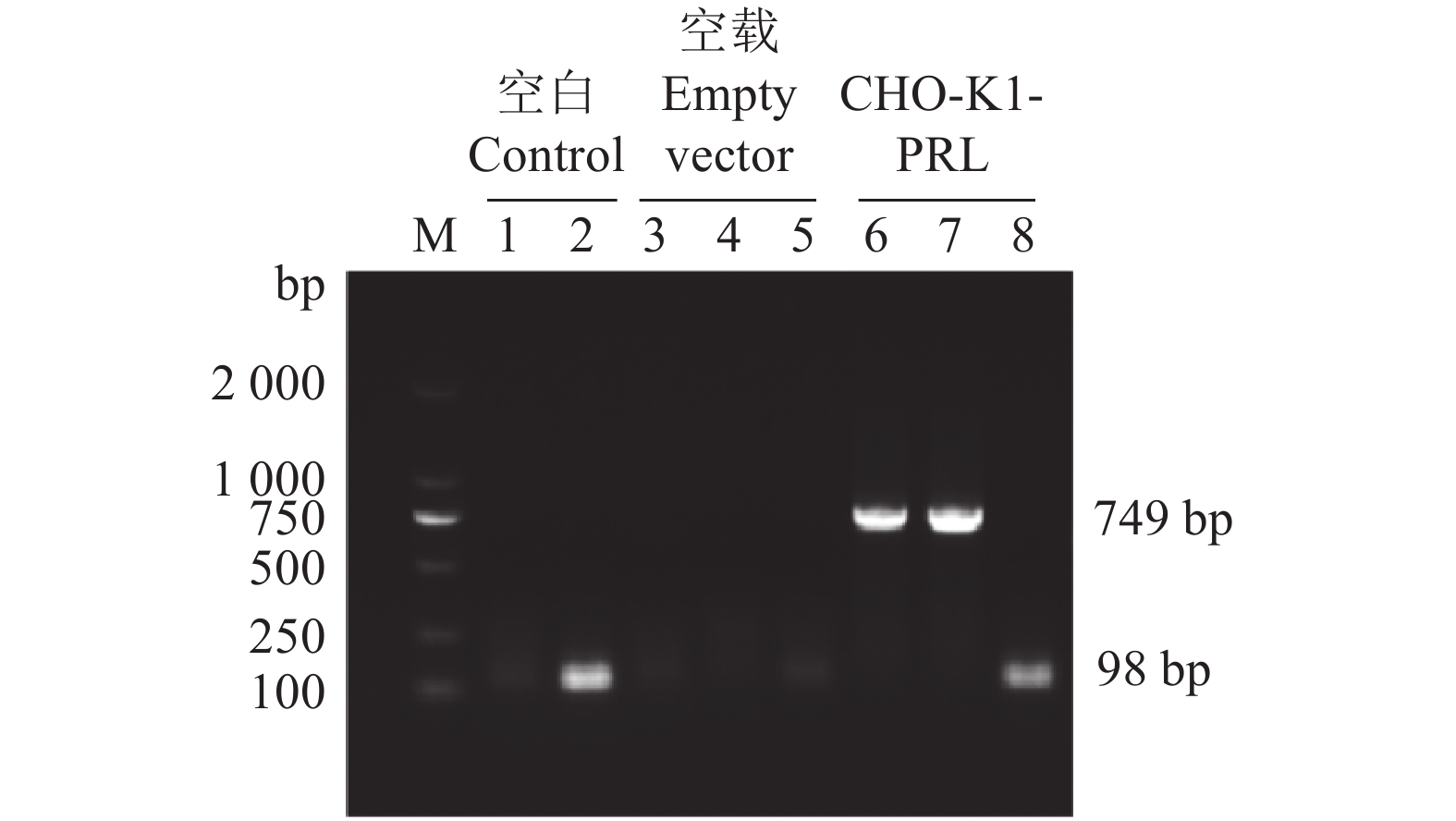
图 5 CHO-K1-PRL细胞的RT-PCR鉴定
M为DL 2000 DNA Marker; 1、3、4、6和7为749 bp 的EcoR I-6His-PRL-6His-BamH I片段,2、5和8为98 bp的β-actin
Figure 5. RT-PCR identification of CHO-K1-PRL cells
M is DL 2000 DNA Marker; 1, 3, 4, 6 and 7 are the 749 bp EcoR I-6His-PRL-6His-BamH I fragments; 2, 5 and 8 are the 98 bp β-actin fragments
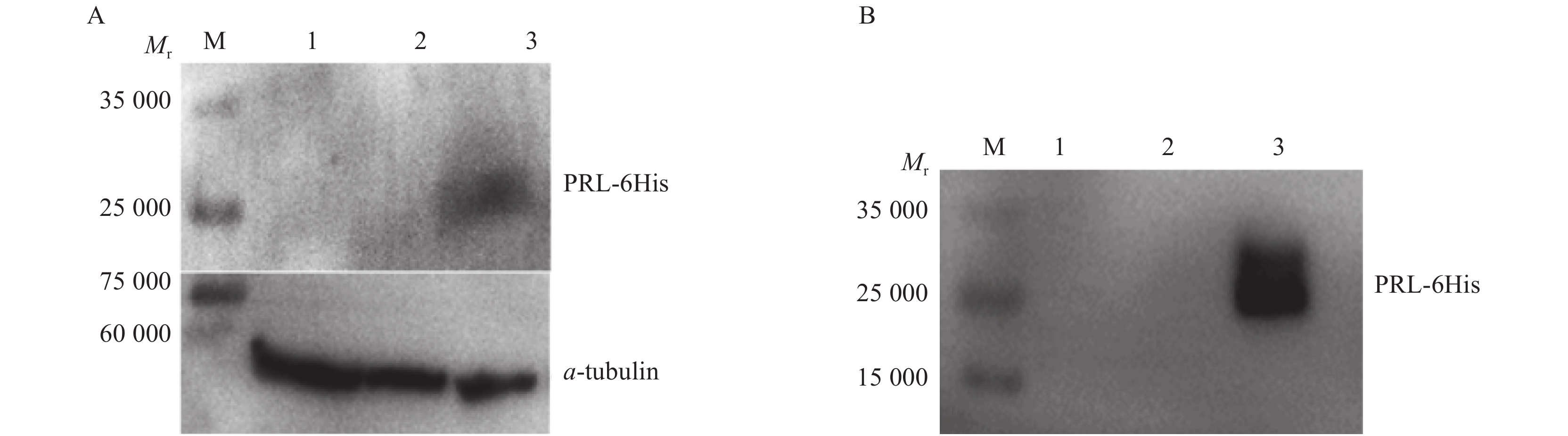
图 6 CHO-K1-PRL细胞中His-Tag蛋白Western blot鉴定
A:细胞裂解液:B:细胞培养液;M为Western blot marker,1为未做处理的CHO-K1细胞,2为感染空载体慢病毒液的CHO-K1细胞,3为CHO-K1-PRL;PRL-6His重组蛋白和内参α-tubulin相对分子质量分别约为25 000和55 000
Figure 6. Western blot identification of His-Tag protein in CHO-K1-PRL cells
A: Cell lysate; B: Cell culture fluid; M is Western blot marker, 1 is untreated CHO-K1 cells, 2 is CHO-K1 cells infected by the concentrated empty lentivirus solution, 3 is CHO-K1-PRL; The relative molecular weights of PRL-6His recombinant protein and α-tubulin are about 25 000 and 55 000 resprectively
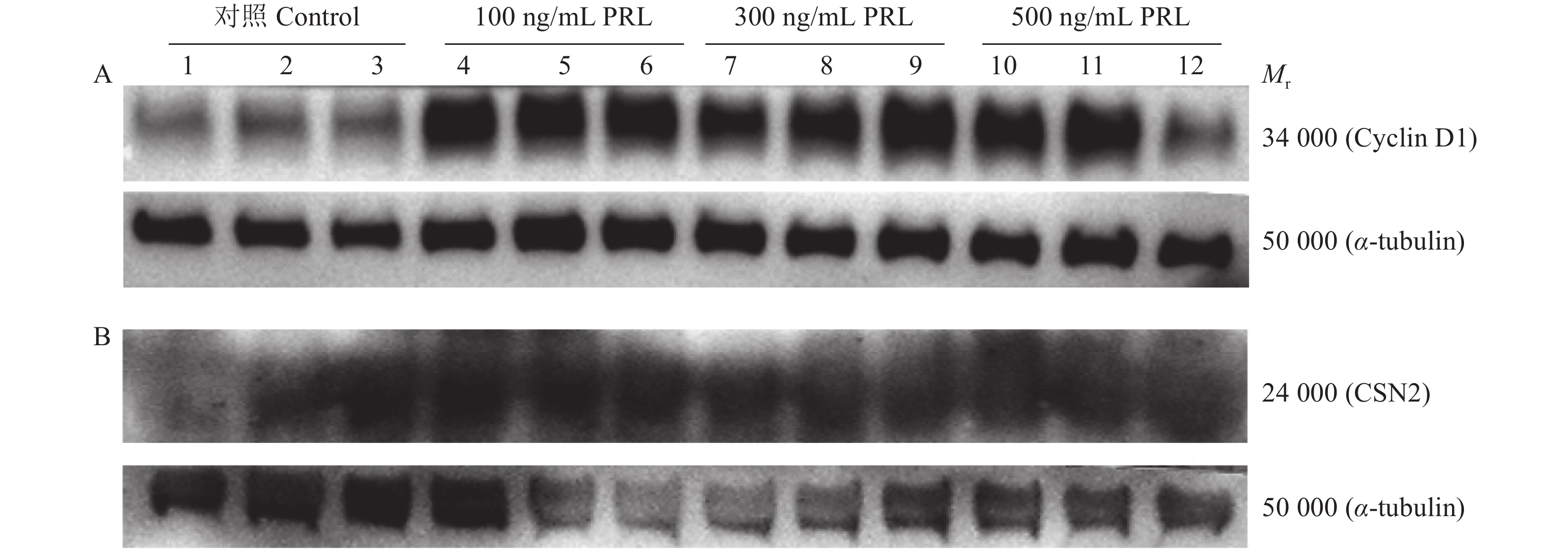
图 8 不同质量浓度重组PRL对HC11细胞增殖及酪蛋白表达的影响
柱子上方(图A)、相同蛋白(图B)或基因(图C)柱子上方的不同小写字母表示组间差异显著(P<0.05,LSD法),不同大写字母表示组间差异极显著(P<0.01,LSD法)
Figure 8. Different mass concentrations of recombinant PRL on proliferation and casein expressions of HC11 cells
Different lowercase letters on bars (figure A), or bars of the same protein (figure B) or gene (figure C) indicate significant differences among groups (P<0.05, LSD method), different capital letters indicate highly significant differences among groups (P<0.01, LSD method)
表 1 qPCR引物序列
Table 1 Primer sequence for qPCR
基因
Gene正向引物序列(5′→ 3′)
Forward primer sequence反向引物序列(5′→ 3′)
Reverse primer sequence产物大小/bp
Product lengthGAPDH GAGCGAGACCCCACTAACATC GCGGAGATGATGACCCTTTT 134 PRLR GTGGAATCCTGGGTCAGATG GGGCCACTGGTTTTGTAGTC 108 CSN1S1 CCTTTCCCCTTTGGGCTTAC TGAGGTGGATGGAGAATGGA 193 CSN2 GCAATCCCGTCCCACAAAAC GGGGCATCTGTTTGTGCTTG 138 CSN3 CCTTTTTGTGCCGTGGTGAG GGCTGGAGACCTAAGCAGAA 197 Cyclin D1 TGGCTAAACAAGGACCCCC ATGTCCACATCTCGCACGTC 203 PCNA AAAGATGCCGTCGGGTGAAT TGGTTACCGCCTCCTCTTCT 179 -
[1] MARANO R J, BEN-JONATHAN N. Minireview: Extrapituitary prolactin: An update on the distribution, regulation, and functions[J]. Molecular Endocrinology, 2014, 28(5): 622-633. doi: 10.1210/me.2013-1349
[2] MACOTELA Y, TRIBEL J, CLAPP C. Time for a new perspective on prolactin in metabolism[J]. Trends in Endocrinology and Metabolism, 2020, 31(4): 276-286. doi: 10.1016/j.tem.2020.01.004
[3] VILAR L, VILAR C F, LYRA R, et al. Pitfalls in the diagnostic evaluation of hyperprolactinemia[J]. Neuroendocrinology, 2019, 109(1): 7-19. doi: 10.1159/000499694
[4] MELMED S, CASANUEVA F F, HOFFMAN A R, et al. Diagnosis and treatment of hyperprolactinemia: An Endocrine Society clinical practice guideline[J]. Journal of Clinical Endocrinology & Metabolism, 2011, 96(2): 273-288.
[5] RATNER L D, GONZALEZ B, AHTIAINEN P, et al. Short-term pharmacological suppression of the hyperprolactinemia of infertile hCG-overproducing female mice persistently restores their fertility[J]. Endocrinology, 2012, 153(12): 5980-5992. doi: 10.1210/en.2012-1393
[6] 何海迎, 王泳, 段春辉, 等. 催乳素对绵羊颗粒细胞雌激素和孕酮分泌及相关基因表达的影响[J]. 中国兽医学报, 2020, 40(11): 2226-2233. [7] PHILLIPPS H R, YIP S H, GRATTAN D R. Patterns of prolactin secretion[J]. Molecular and Cellular Endocrinology, 2020, 502: 110679. doi: 10.1016/j.mce.2019.110679
[8] SAMPERI I, LITHGOW K, KARAVITAKI N. Hyperprolactinaemia[J]. Journal of Clinical Medicine, 2019, 8(12): 2203.
[9] DI FILIPPO L, DOGA M, RWSMINI E, et al. Hyperprolactinemia and bone[J]. Pituitary, 2020, 23(3): 314-321. doi: 10.1007/s11102-020-01041-3
[10] BASINI G, BAIONI L, BUSSOLATI S, et al. Prolactin is a potential physiological modulator of swine ovarian follicle function[J]. Regulatory Peptides, 2014, 189: 22-30. doi: 10.1016/j.regpep.2014.01.003
[11] TRIEBEL J, BERTSCH T, BOLLHEIMER C, et al. Principles of the prolactin/vasoinhibin axis[J]. American Journal of Physiology Regulatory Integrative & Comparative Physiology, 2015, 309(10): R1193-R1203.
[12] TRIEBEL J, ROBLES-OSORIO M L, GARCIA-FRANCO R, et al. From bench to bedside: Translating the prolactin/vasoinhibin axis[J]. Frontiers in Endocrinology, 2017, 8: 342. doi: 10.3389/fendo.2017.00342
[13] SINHA Y N. Structural variants of prolactin: Occurrence and physiological significance[J]. Endocrine Reviews, 1995, 16(3): 354-369. doi: 10.1210/edrv-16-3-354
[14] 田青, SPITZER A J, ZHAO F. PRL对HC11细胞乳蛋白及其调节因子基因表达的影响[J]. 中国乳品工业, 2020, 48(12): 20-23. doi: 10.19827/j.issn1001-2230.2020.12.004 [15] 田青, 王洪荣. 胰岛素、催乳素和氢化可的松对奶牛乳腺上皮细胞增殖和凋亡的影响[J]. 中国饲料, 2013(2): 8-12. [16] NUC P, NUC K. Recombinant protein production in Escherichia coli[J]. Postepy Biochemii, 2006, 52(4): 448-456.
[17] 邓春梅, 葛玉强, 刘丽, 等. 外源基因表达系统的研究进展[J]. 现代生物医学进展, 2010, 10(19): 3744-3746. [18] KIM J Y, KIM Y G, LEE G M. CHO cells in biotechnology for production of recombinant proteins: Current state and further potential[J]. Applied Microbiology and Biotechnology, 2012, 93(3): 917-930. doi: 10.1007/s00253-011-3758-5
[19] JAIN N K, BARKOWSLI-CLARK S, ALTMAN R, et al. A high density CHO-S transient transfection system: Comparison of ExpiCHO and Expi293[J]. Protein Expression & Purification, 2017, 134: 38-46.
[20] 孟凡荣, 陈琛, 万海粟, 等. 慢病毒载体及其研究进展[J]. 中国肺癌杂志, 2014, 17(12): 870-876. [21] COCKRELL A S, KAFRI T. Gene delivery by lentivirus vectors[J]. Molecular Biotechnology, 2007, 36(3): 184-204. doi: 10.1007/s12033-007-0010-8
[22] FOLLENZI A, SANTAMBROGIO L, ANNONI A. Immune responses to lentiviral vectors[J]. Current Gene Therapy, 2007, 7(5): 306-315. doi: 10.2174/156652307782151515
[23] ALARCON H, BONZON-KULICHENKO E, PEINADO R, et al. Generation of a lentiviral vector system to efficiently express bioactive recombinant human prolactin hormones[J]. Molecular and Cellular Endocrinology, 2020, 499: 110605. doi: 10.1016/j.mce.2019.110605
[24] 宋倩倩, 王文玲, 詹瑛, 等. 真核表达MERS-CoV刺突蛋白亚单位的信号肽序列优化研究[J]. 病毒学报, 2019, 35(1): 20-26. [25] 董金蓉, 毛树宝, 谢震渊, 等. 人巨细胞病毒gB/AD1基因在CHO细胞中的表达和纯化[J]. 生命科学研究, 2015, 19(5): 402-409. [26] FARMER C. Prolactin and the swine mammary gland[J]. Domestic Animal Endocrinology, 2022, 78: 106672. doi: 10.1016/j.domaniend.2021.106672
[27] FREEMAN M E, KANYICSKA B, LERANT A, et al. Prolactin: Structure, function, and regulation of secretion[J]. Physiological Reviews, 2000, 80(4): 1523-1631. doi: 10.1152/physrev.2000.80.4.1523
[28] AKERS R M. Major advances associated with hormone and growth factor regulation of mammary growth and lactation in dairy cows[J]. Journal of Dairy Science, 2006, 89(4): 1222-1234. doi: 10.3168/jds.S0022-0302(06)72191-9
[29] LEE G Y, KENNY P A, LEE E H, et al. Three-dimensional culture models of normal and malignant breast epithelial cells[J]. Nature Methods, 2007, 4(4): 359-365. doi: 10.1038/nmeth1015



 下载:
下载: