Effect of initiation codon mutation within tva receptor gene on chicken resistance to infection by avian leukemia virus subgroup A
-
摘要:目的
探索tva受体基因起始密码子突变(tva c.3G>A)对鸡感染A 亚群禽白血病病毒(Avian leukemia virus subgroup A, ALV-A)的影响。
方法利用Sanger 测序和RT-PCR验证我国黄羽肉鸡存在tva c.3G>A突变。利用流式细胞术检测tva c.3G>A突变对鸡体外感染RCASBP(A)-GFP荧光报告病毒的影响。通过ALV-A体内攻毒试验,探究tva c.3G>A突变对鸡体内感染ALV-A的影响。利用直接测序方法对我国黄羽肉鸡品系tva c.3G>A突变位点进行基因分型。
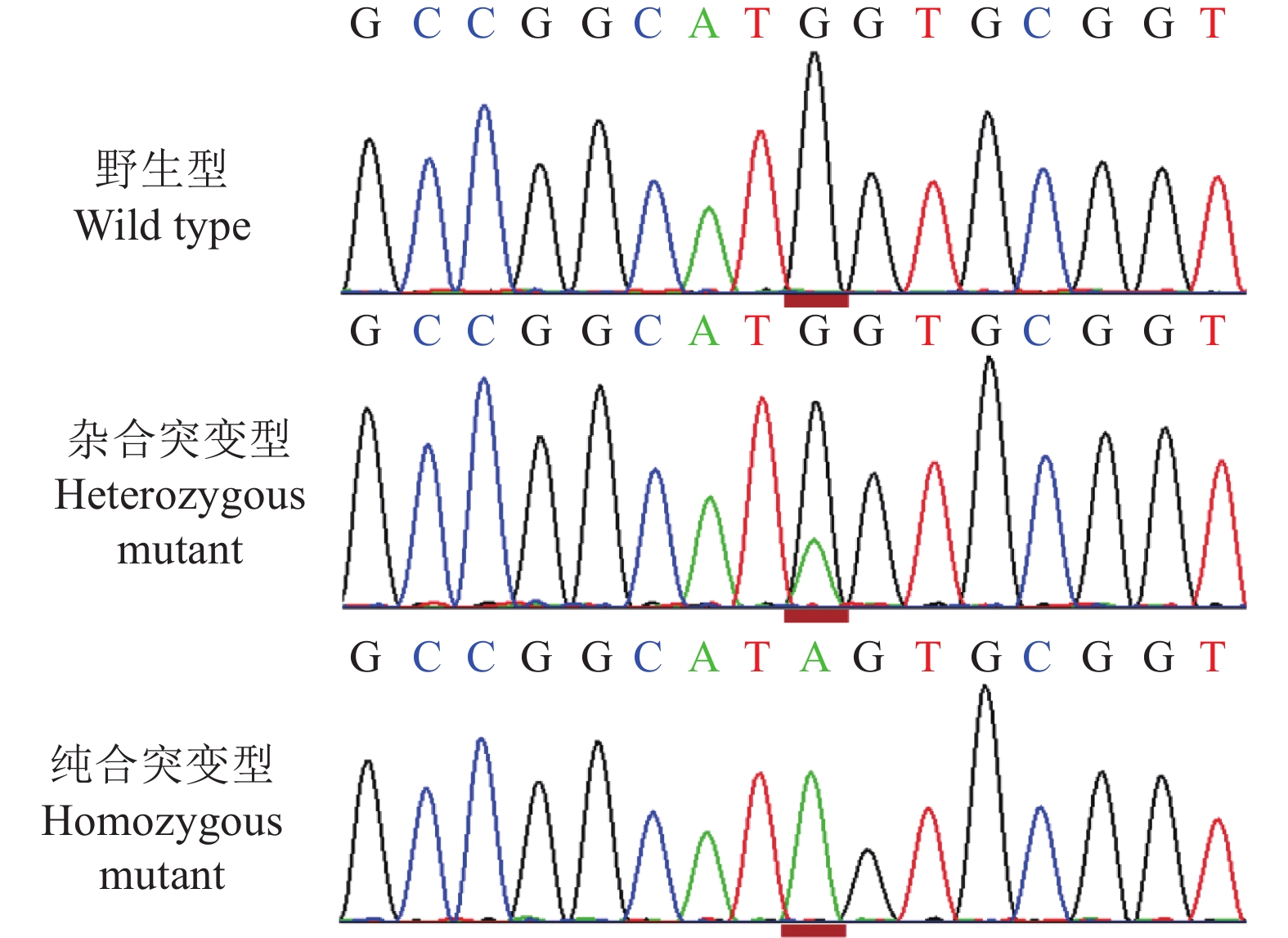
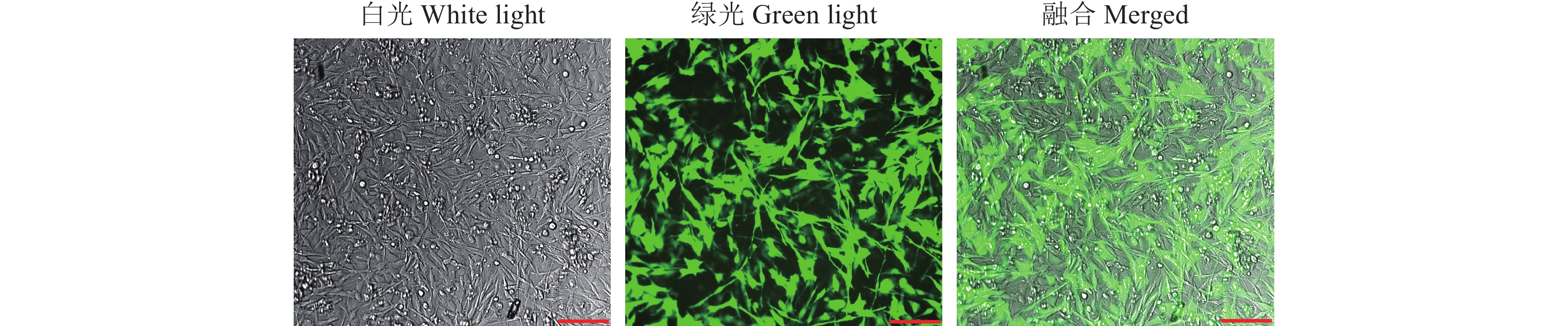
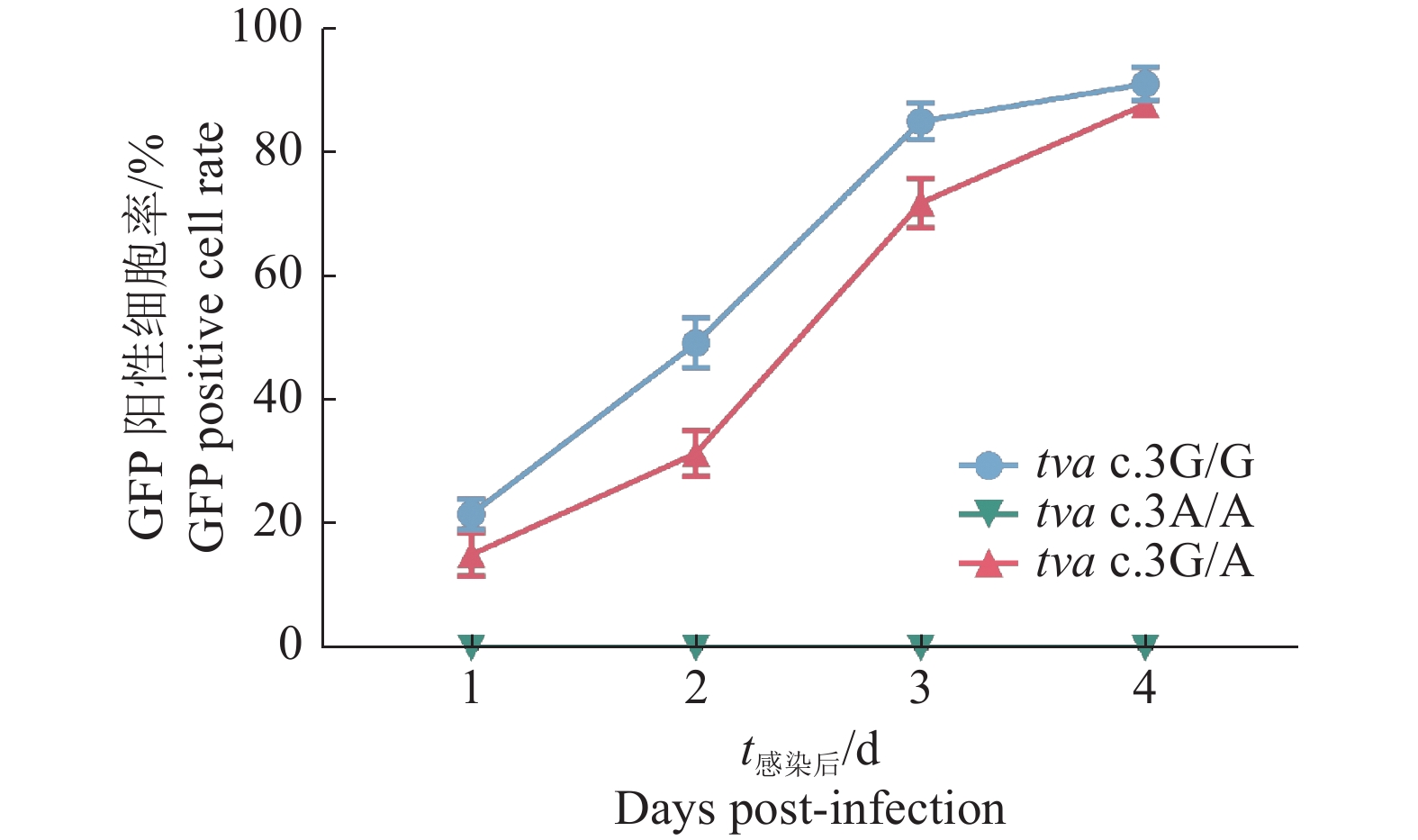
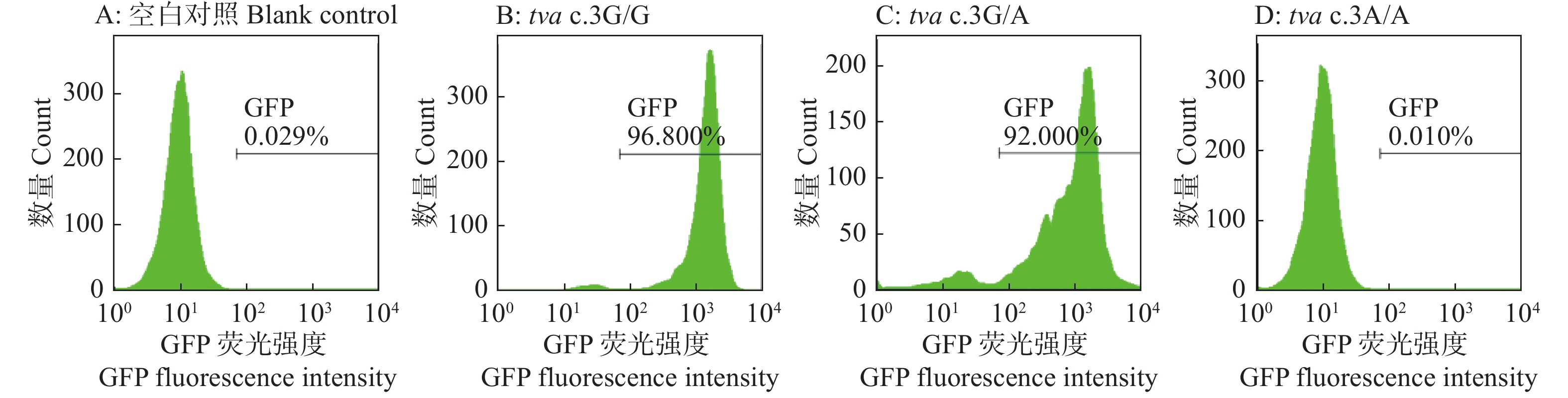
结果Sanger测序和RT-PCR结果鉴定我国黄羽肉鸡品系tva基因编码区第3位碱基由G突变为A,该突变引起tva基因起始密码子序列由ATG突变为ATA。流式细胞术检测结果显示野生型tva c.3G/G鸡胚成纤维细胞(Chicken embryo fibroblast, CEF)对RCASBP(A)-GFP易感,纯合突变型tva c.3A/A CEF抗RCASBP(A)-GFP感染,表明tva c.3G>A突变导致鸡体外抗RCASBP(A)-GFP的感染。ALV-A体内攻毒试验的结果表明,tva c.3G>A突变导致鸡体内抗ALV-A的感染。tva c.3G>A突变位点基因分型发现,CB01、CB08、CB10和CB15品系存在纯合抗性基因型tva c.3A/A,频率分别为0.10、0.15、0.23和0.08。
结论tva c.3G>A突变引起鸡在体外、体内抗ALV-A感染,tva c.3G>A突变位点可作为ALV-A的遗传抗性位点。
Abstract:ObjectiveTo explore the effect of initiation codon mutation within tva receptor gene (tva c.3G>A) on resistance of chickens to infection by avian leukemia virus subgroup A (ALV-A).
MethodSanger sequencing and RT-PCR were used to verify the presence of tva c.3G>A mutation in Chinese yellow-feathered broilers. The effect oftva c.3G>A mutation on infection of chickens by RCASBP(A)-GFP fluorescence reporter virusin vitro was detected using flow cytometry. The effect of tva c.3G>A mutation on infection of chickens by ALV-A was investigated using ALV-A challenge testin vivo. Direct sequencing was used to genotypetva c.3G>A mutation site within Chinese yellow-feathered broiler lines.
ResultThe results of Sanger sequencing and RT-PCR identified the mutation from G to A on the third base in the coding region of tva gene of Chinese yellow-feathered broilers, which caused the mutation from ATG to ATA in the initial codon sequence of tva gene. The result of flow cytometry showed that chicken embryo fibroblasts (CEFs) of wild-type tva c.3G/G were susceptible to infection by RCASBP(A)-GFP, while the homozygous mutant tva c.3A/A CEFs were resistant to infection by RCASBP(A)-GFP, indicating that tva c.3G>A mutation led to chicken resistance to infection by RCASBP(A)-EGFPin vitro. The results of ALV-A challenge test in vivo also indicated that tva c.3G>A mutation led to chicken resistance to infection by ALV-A. Genotyping oftva c.3G>A revealed that homozygous resistance genotypetva c.3A/A was present in lines CB01, CB08, CB10 and CB15, with the frequencies of 0.10, 0.15, 0.23 and 0.08, respectively.
ConclusionThe tva c.3G>A mutation causes chicken resistance to infection by ALV-Ain vitro and in vivo, and the tva c.3G>A mutation site can be used as the genetic resistance site of ALV-A.
-
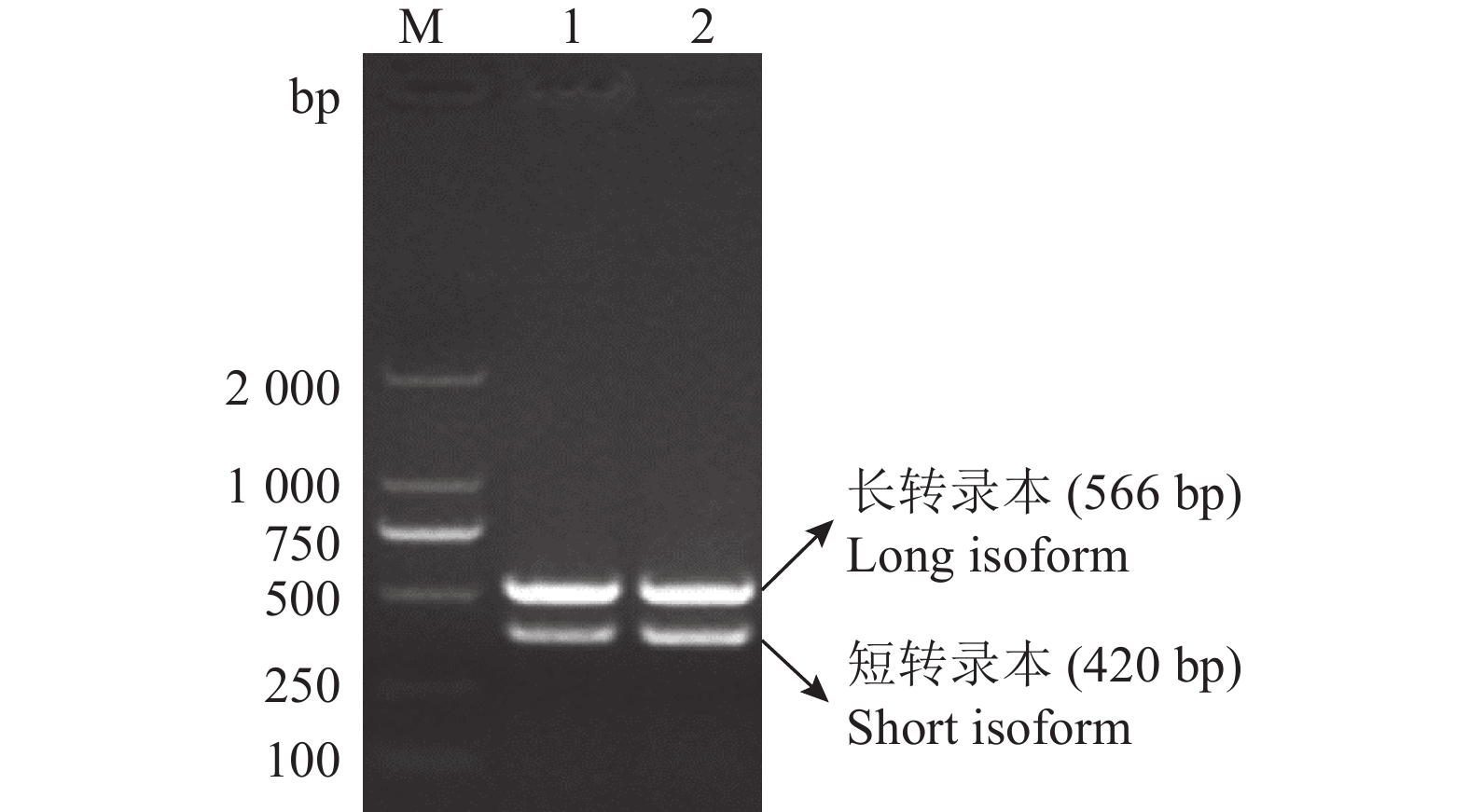
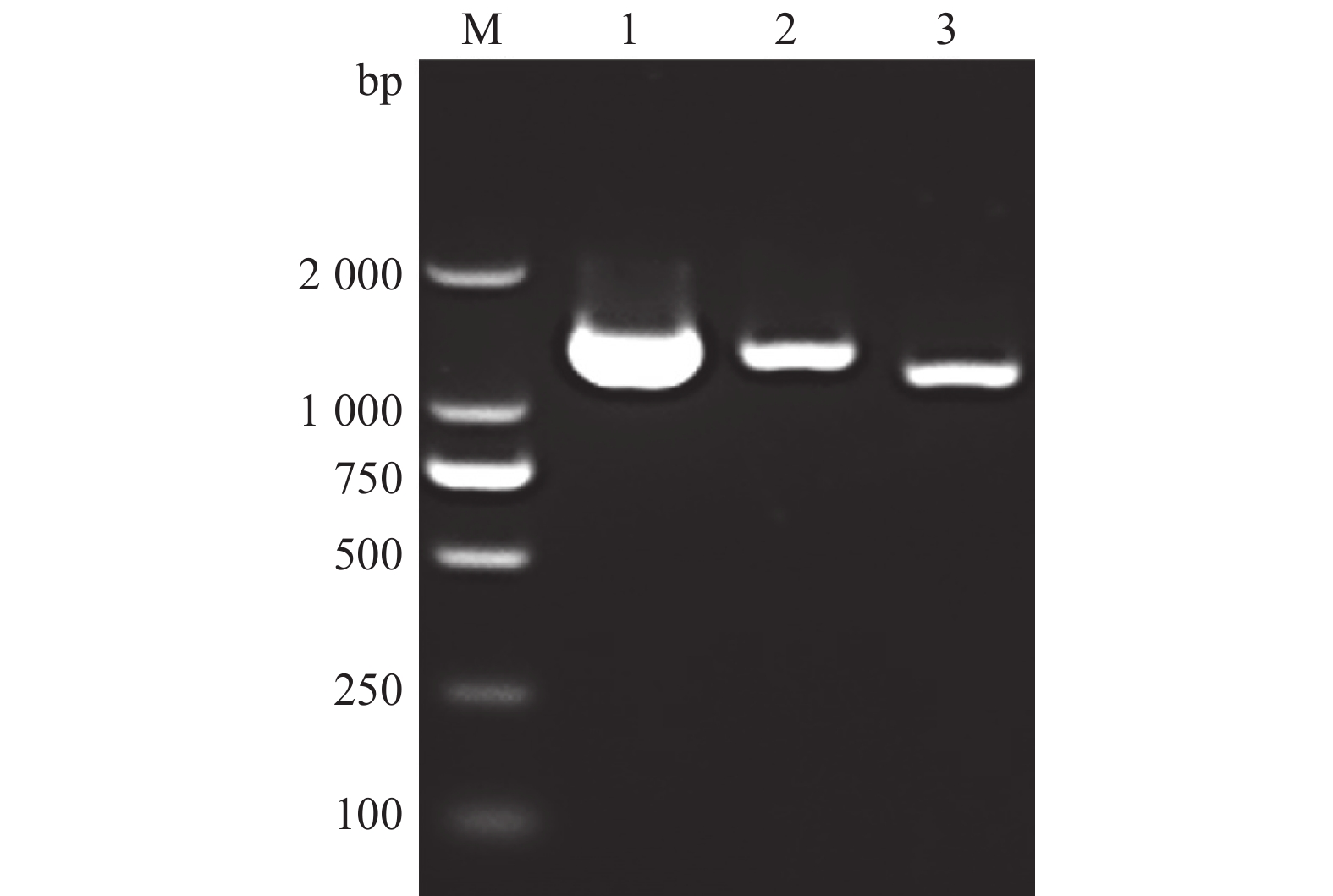
图 3 tva c.3G>A突变位点不同基因型血样全长tva编码序列的RT-PCR扩增结果
M:DL2000 marker;1:野生型tva c.3G/G;2:纯合突变型tva c.3A/A
Figure 3. RT-PCR amplified results of the full-lengthtva coding sequences for blood samples from different genotypes of tva c.3G>A mutation site
M: DL2000 marker; 1: Wild type tva c.3G/G; 2: Homozygous mutant tva c.3A/A
表 1 tva受体基因全长序列PCR扩增引物信息
Table 1 Primers used to amplify the whole sequence of tva receptor gene
片段 Fragment 引物名称 Primer name 引物序列(5′→3′) Primer sequence 退火温度/℃ Annealing temperature 片段大小/bp Segment size 1 P1-F GTTCAGCAGATCCTCATCTCCCG 62 1308 P1-R GGCCATTGTGCGATCTAAGAGGG 2 P2-F AGCCCTCTTAGATCGCACAA 60 1253 P2-R GTGACACCGAGCACAAAATG 3 P3-F GTTGGAGCTGGATGAGCACT 60 1132 P3-R TGAGGGAATTCCTGTCACCT 表 2 ALV-A攻毒后雏鸡病毒血症阳性率
Table 2 Positive infection rate of viremia in chicks infected by ALV-A
雏鸡 Chick 基因型 Genotype 阳性样品数/总样品数 No. of positive samples/Total No. of samples 阳性感染率/% Positive infection rate SPF tva c.3G/G 9/9 100 CB06 tva c.3G/G 25/25 100 tva c.3G/A 21/28 75 tva c.3A/A 0/22 0 表 3 我国黄羽肉鸡品系 tva c.3G>A抗性位点的基因型频率分布
Table 3 Genotypic frequency of tva c.3G>A resistance locus in Chinese yellow-feathered broiler lines
品系 Line 样品数/只 No. of samples 基因型 Genotype tva c.3G/G tva c.3G/A tva c.3A/A CB01 60 0.85 0.05 0.10 CB02 50 1 0 0 CB03 36 1 0 0 CB04 30 1 0 0 CB05 48 1 0 0 CB06 60 1 0 0 CB07 30 1 0 0 CB08 60 0.80 0.05 0.15 CB09 30 1 0 0 CB10 60 0.67 0.10 0.23 CB11 35 1 0 0 CB12 45 1 0 0 CB13 60 1 0 0 CB14 30 1 0 0 CB15 36 0.78 0.14 0.08 -
[1] 李德娟, 崔梦笛, 王学静, 等. 禽白血病病毒及其受体研究进展[J]. 黑龙江畜牧兽医, 2022(9): 34-41. [2] 王鑫, 赵鹏, 崔治中. 我国地方品种鸡分离到的一个禽白血病病毒新亚群的鉴定[J]. 病毒学报, 2012, 28(6): 609-614. doi: 10.13242/j.cnki.bingduxuebao.002329 [3] PAYNE L N, NAIR V. The long view: 40 years of avian leukosis research[J]. Avian Pathology, 2012, 41(1): 11-19. doi: 10.1080/03079457.2011.646237
[4] 崔治中. 禽白血病病毒研究的过去、现在和将来[J]. 生命科学, 2012, 24(4): 305-309. doi: 10.13376/j.cbls/2012.04.001 [5] 崔治中. 种鸡场禽白血病防控和净化技术方案[J]. 中国家禽, 2015, 37(23): 1-7. doi: 10.16372/j.issn.1004-6364.2015.23.001 [6] 俞燕. 地方品种鸡群禽白血病流行病学调查及检测净化技术研究[D]. 扬州: 扬州大学, 2019. [7] SU Q, LI Y, LI W, et al. Molecular characteristics of avian leukosis viruses isolated from indigenous chicken breeds in China[J]. Poultry Science, 2018, 97(8): 2917-2925. doi: 10.3382/ps/pex367
[8] 冯敏, 谭利强, 代曼曼, 等. 种禽场A亚群禽白血病病原学调查及分离株遗传进化分析[J]. 华南农业大学学报, 2014, 35(4): 11-15. doi: 10.7671/j.issn.1001-411X.2014.04.003 [9] 钱琨, 朱钰峰, 沈海玉, 等. 地方蛋鸡群A亚群禽白血病病毒的分离与全基因组序列分析[J]. 中国兽医科学, 2011, 41(10): 1005-1010. [10] 杨波, 高玉龙, 高宏雷, 等. 我国东北地区野生鸟类A亚群禽白血病病毒分子流行病学调查及env基因序列分析[J]. 中国预防兽医学报, 2013, 35(3): 245-247. doi: 10.3969/j.issn.1008-0589.2013.03.18 [11] BACON L D, HUNT H D, CHENG H H. A review of the development of chicken lines to resolve genes determining resistance to diseases[J]. Poultry Science, 2000, 79(8): 1082-1093. doi: 10.1093/ps/79.8.1082
[12] JIE H, LIU Y P. Breeding for disease resistance in poultry: Opportunities with challenges[J]. World’s Poultry Science Journal, 2011, 67(4): 687-696. doi: 10.1017/S0043933911000766
[13] CHENG H H, KAISER P, LAMONT S J. Integrated genomic approaches to enhance genetic resistance in chickens[J]. Annual Review of Animal Biosciences, 2013, 1: 239-260. doi: 10.1146/annurev-animal-031412-103701
[14] BATES P, YOUNG J A, VARMUS H E. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor[J]. Cell, 1993, 74(6): 1043-1051. doi: 10.1016/0092-8674(93)90726-7
[15] BARNARD R J, ELLEDER D, YOUNG J A. Avian sarcoma and leukosis virus-receptor interactions: From classical genetics to novel insights into virus-cell membrane fusion[J]. Virology, 2006, 344(1): 25-29. doi: 10.1016/j.virol.2005.09.021
[16] ELLEDER D, MELDER D C, TREJBALOVA K, et al. Two different molecular defects in the Tva receptor gene explain the resistance of two tvar lines of chickens to infection by subgroup A avian sarcoma and leukosis viruses[J]. Journal of Virology, 2004, 78(24): 13489-13500. doi: 10.1128/JVI.78.24.13489-13500.2004
[17] REINISOVA M, PLACHY J, TREJBALOVA K, et al. Intronic deletions that disrupt mRNA splicing of the tva receptor gene result in decreased susceptibility to infection by avian sarcoma and leukosis virus subgroup A[J]. Journal of Virology, 2012, 86(4): 2021-2030. doi: 10.1128/JVI.05771-11
[18] CHEN W, LIU Y, LI H, et al. Intronic deletions of tva receptor gene decrease the susceptibility to infection by avian sarcoma and leukosis virus subgroup A[J]. Scientific Reports, 2015, 5: 9900. doi: 10.1038/srep09900
[19] LIAO C T, CHEN S Y, CHEN W G, et al. Single nucleotide polymorphism variants within tva and tvb receptor genes in Chinese chickens[J]. Poultry Science, 2014, 93(10): 2482-2489. doi: 10.3382/ps.2014-04077
[20] KREAGER K S. Chicken industry strategies for control of tumor virus infections[J]. Poultry Science, 1998, 77(8): 1213-1216. doi: 10.1093/ps/77.8.1213
[21] MENG F, LI Q, ZHANG Y, et al. Characterization of subgroup J avian leukosis virus isolated from Chinese indigenous chickens[J]. Virology Journal, 2018, 15(1): 33. doi: 10.1186/s12985-018-0947-1
[22] LI H, TAN M, ZHANG F, et al. Diversity of Avian leukosis virus subgroup J in local chickens, Jiangxi, China[J]. Scientific Reports, 2021, 11: 4797. doi: 10.1038/s41598-021-84189-7
[23] ADKINS H B, BLACKLOW S C, YOUNG J A. Two functionally distinct forms of a retroviral receptor explain the nonreciprocal receptor interference among subgroups B, D, and E avian leukosis viruses[J]. Journal of Virology, 2001, 75(8): 3520-3526. doi: 10.1128/JVI.75.8.3520-3526.2001
[24] ELLEDER D, STEPANETS V, MELDER D C, et al. The receptor for the subgroup C avian sarcoma and leukosis viruses, Tvc, is related to mammalian butyrophilins, members of the immunoglobulin superfamily[J]. Journal of Virology, 2005, 79(16): 10408-10419. doi: 10.1128/JVI.79.16.10408-10419.2005
[25] CHAI N, BATES P. Na/H exchanger type 1 is a receptor for pathogenic subgroup J avian leukosis virus[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(14): 5531-5536. doi: 10.1073/pnas.0509785103
[26] LI X, CHEN W, ZHANG H, et al. Naturally occurring frameshift mutations in the tvb receptor gene are responsible for decreased susceptibility of chicken to infection with avian leukosis virus subgroups B, D, and E[J]. Journal of Virology, 2018, 92(8): e01770-e01717.
-
期刊类型引用(12)
1. 刘玉茹,李欣怡,陈晓民,张淑丽,张德喜. 饲料种类与生牛乳豆腥味相关性. 乳业科学与技术. 2025(02): 20-29 .  百度学术
百度学术
2. 董闯,续辉,祝亚辉,杨林. 基于HS-SPME-GC-MS和OAV分析西藏不同地区牦牛乳拉拉关键呈香物质. 食品工业科技. 2025(10): 281-290 .  百度学术
百度学术
3. 项雅科,杨梦蝶,孙海斓,席嘉佩,陈潇,赵立艳. 2种鲜食黑糯玉米营养组分及香气的比较. 中国粮油学报. 2024(01): 196-203 .  百度学术
百度学术
4. 童丹,原霁虹,孙永军. 紫色马铃薯糕加工工艺参数优化. 湖北农业科学. 2024(05): 147-150+240 .  百度学术
百度学术
5. 阳晖,彭秀分,余思瑾,黄洁,豆念,罗雪,李昌满,宁诗颢. 发酵法脱除萝卜红色素异味的菌种筛选. 食品研究与开发. 2023(09): 144-151 .  百度学术
百度学术
6. 谢雪华,邱月,王旭骅,许蜜蜜,张建友,丁玉庭,吕飞. 基于QDA和GC-MS的热加工牛肉特征挥发性风味物质分析. 中国食品学报. 2023(05): 301-310 .  百度学术
百度学术
7. 周志帅,李娇,林德贤,何利. HS-SPME-GC-MS结合OAV分析不同产地青花椒浸提前、后的关键香气成分. 中国食品学报. 2023(10): 315-325 .  百度学术
百度学术
8. 阳晖,陈明月,龚乃霞,曹沁倩,张川雨,李昌满. 微生物发酵法脱除胭脂萝卜红色素异味的工艺优化. 食品工业. 2022(07): 57-62 .  百度学术
百度学术
9. 李娇,周志帅,申光辉. 美拉德反应改善熟化马铃薯冻融酶解汁液风味. 中国食品学报. 2022(11): 190-203 .  百度学术
百度学术
10. 闫晨苗,王玺,段盛林,李铁梅,苑鹏,林静,崔立柱,夏凯,刘美玉. 不同干燥方式对马铃薯全粉糊化特性、风味及薯粉面包品质的影响. 食品工业科技. 2020(09): 34-41 .  百度学术
百度学术
11. 洪森荣,刘洁,曾欣怡,蔡红,陈荣华. 怀玉山高山马铃薯及其平原种植退化块茎的转录组分析. 西南师范大学学报(自然科学版). 2020(11): 157-166 .  百度学术
百度学术
12. 王玺,闫晨苗,李铁梅,苑鹏,万宁,段盛林. 马铃薯粉粒度对面包风味及品质的影响. 食品工业. 2020(12): 137-141 .  百度学术
百度学术
其他类型引用(7)



 下载:
下载: