Characterization, identification and functional analysis of miRNA in seminal plasma exosomes of Yorkshire boar
-
摘要:目的
精浆外泌体(Seminal plasma exosome,spEX)在精子成熟、凋亡、受精中起着至关重要的作用。本文旨在探究种公猪spEX miRNA表达及miRNA在精子成熟及功能维持过程中的潜在调控作用。
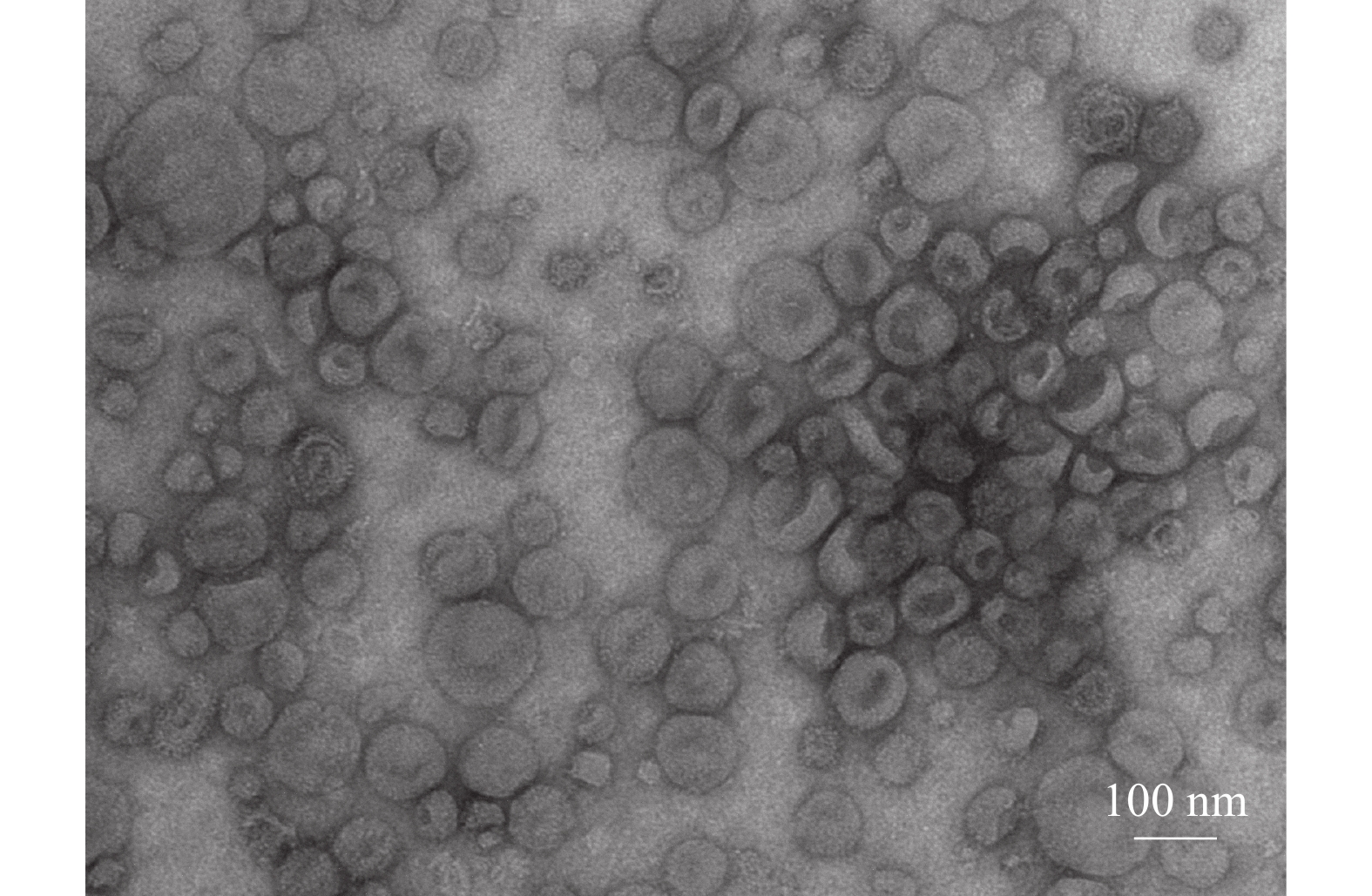
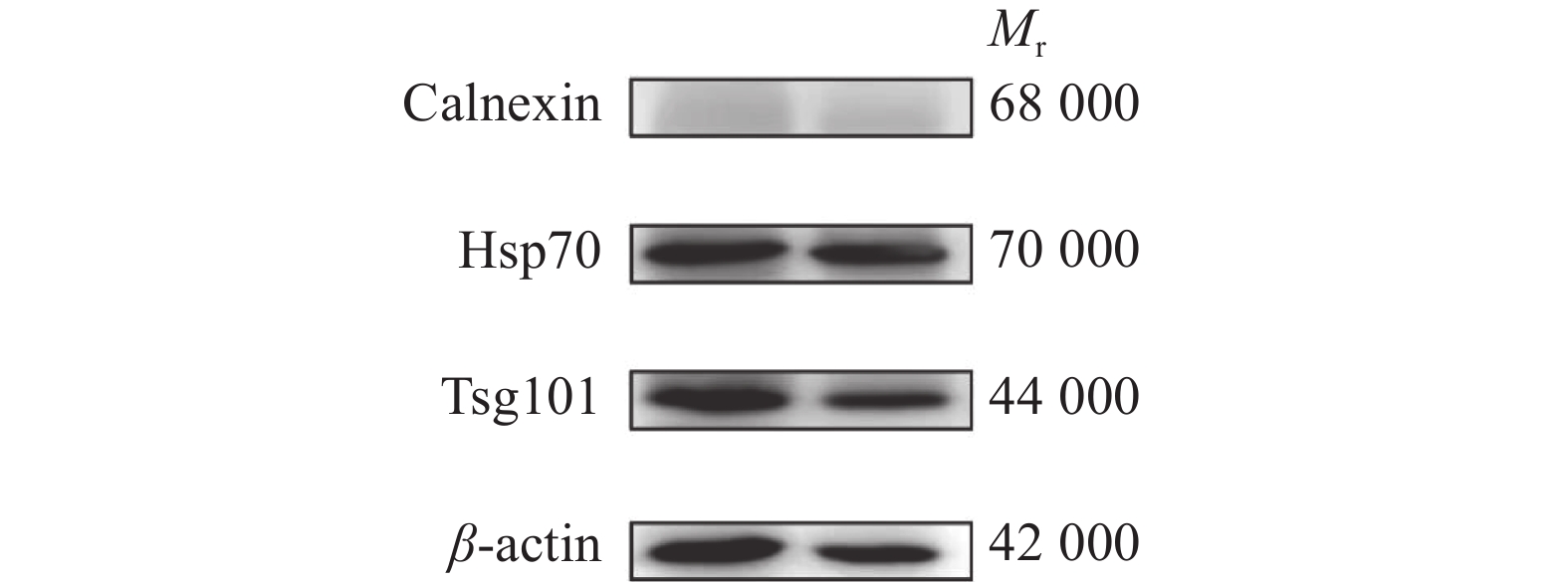
方法提取大白公猪spEX并进行电镜分析、粒径分析、标志性蛋白表达分析和miRNA高通量测序。
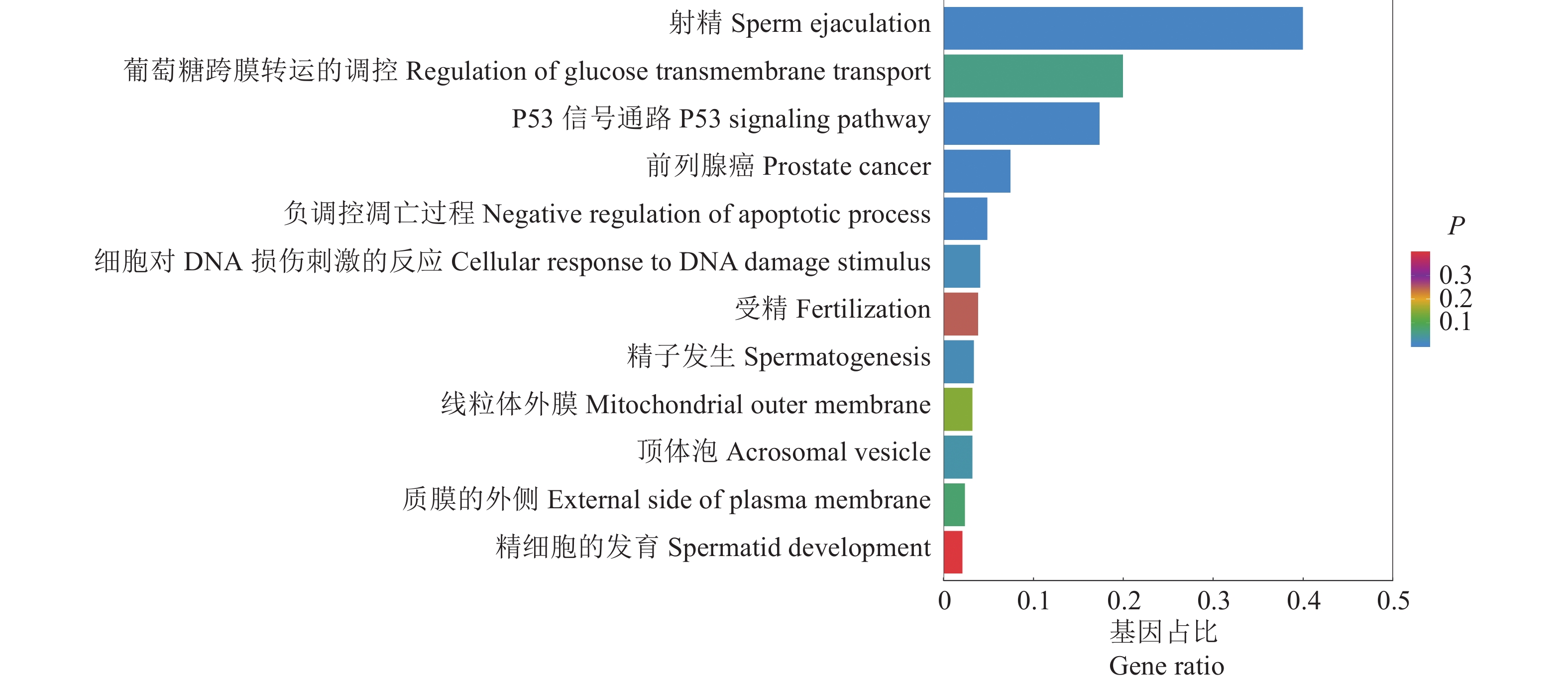
结果成功分离出spEX,利用miRNA测序共鉴定出329个spEX miRNA。对高表达miRNA进行靶基因预测和功能富集分析,结果表明spEX miRNA在射精、P53信号通路、前列腺癌、细胞对DNA损伤刺激的反应、负调控凋亡过程、顶体膜结合、受精等通路中均发挥了潜在调控作用。
结论本研究为spEX miRNA在调控精子活力和精子受精作用方面提供基础数据,并为精液保存调控机制的研究提供参考。
Abstract:ObjectiveSeminal plasma exosomes (spEXs) play a crucial role in sperm maturation, apoptosis and fertilization. This study was aimed to explore the miRNA expression of boar spEXs and the potential regulatory role of miRNA in sperm maturation and functional maintenance.
MethodThe spEXs from Yorkshire boar semen were isolated and the exosomes were identified through transmission electron microscopy, nanoparticle tracking analysis, marker protein expression analysis and high-throughput miRNA sequencing.
ResultThe spEXs were successfully isolated. A total of 329 spEX miRNAs were identified by miRNA sequencing. Through target gene prediction and functional enrichment analysis of highly expressed miRNAs, it was concluded that spEX miRNAs played potential regulatory roles in sperm ejaculation, P53 signaling pathway, prostate cancer, cell response to DNA damage stimuli, negative regulation of apoptosis, acrosome membrane binding, fertilization, etc.
ConclusionThis study provides basic data for spEX miRNAs regulating sperm motility and sperm fertilization, and provides references for studying the regulation mechanism of semen preservation.
-
Keywords:
- Seminal plasma exosome /
- Yorkshire boar /
- miRNA /
- Target gene /
- Sperm motility /
- Sperm apoptosis
-
动物的采食行为是维持机体能量稳态的基础,畜禽生产中获得充足的食物是其生长发育的前提。动物采食量受中枢调控,其中胃肠道状态是决定畜禽食欲的关键部位。揭示饥饿状态下鸡食欲调控的潜在肠−脑轴机制可为如何提高鸡采食量提供理论依据。下丘脑弓状核作为食欲调控中枢[1-2]存在大量的促采食的刺鼠相关蛋白(Agouti-related protein,AgRP)/神经肽 Y(Neuropeptide Y,NPY)神经元和抑采食的前阿片黑色皮质素(Proopiomelanocortin,POMC)/可卡因−苯丙胺调节转录肽(Cocaine and amphetamine regulated transcript,CART)神经元[3-6]。影响动物食欲的因素有很多,遗传、环境因素、机体健康以及肠道充盈状态等均能影响动物采食量[7]。其中胃肠道作为营养物质暂时储存和消化吸收的关键部位,存在大量食欲调控信号[8]。这些食欲调控信号一方面通过血液循环被中枢所感应[2, 9],另一方面被肠道迷走感觉传入神经元直接感应,经脑干孤束核最终将信号投递至食欲调控中枢,肠道和中枢间的这种信息传递被称为“肠−脑轴” [8, 10]。肠道迷走感觉神经作为假单极双向神经元,位于结状神经节处的胞体分别向中枢孤束核和肠道发出轴突,其中肠道迷走神经末梢存在多种受体感应肠道各种理化信号,例如游离脂肪酸受体2 (FFAR2)、生长激素促分泌素受体(GHSR)、胆囊收缩素受体(CCKR),以及炎症受体TLR4等 [10-12]。
肠道健康对机体维持高食欲具有重要作用,维持肠道平衡可以维持机体正常食欲,反之肠道菌群紊乱等原因导致肠道健康受损则会引起采食量降低[13-14],而肠道屏障是肠道发挥其正常生物学功能的重要前提[15-16],肠道黏膜屏障包括肠上皮细胞及胞间连接,其中,紧密连接是肠上皮细胞间的细胞旁通路的主要屏障,闭合(Occludin)和紧密连接(Claudin)家族成员是影响其功能的主要封闭蛋白,二者与胞浆蛋白相互作用维持紧密蛋白的完整性[17-18]。当肠道出现炎症时,会导致Claudin蛋白结构变化,进而引起肠道屏障功能性障碍,并且受致病菌侵害也会导致肠道屏障通透性增加[19];动物炎症性肠病会导致肠道隐窝改变、小肠绒毛萎缩或变平以及一系列的形态学变化[20]。大量研究发现间歇性饥饿有助于维持肠道及肠道屏障的完整性[21-22]。
此外,胃肠道中上皮基质和微生物群落共调控生成活性氧,导致生成H2O2;而H2O2是维持正常细胞稳态和生理功能所必需的第二信使[23]。Miller等[24]研究发现,结肠内壁中的细胞会释放H2O2(而非氧气)来限制微生物的生长,H2O2可以协同其他物质在肠道黏膜上形成保护,防止菌群紊乱或肠道炎症对机体造成损伤,并且可以治疗肠道炎症,恢复机体正常生理功能。然而,目前并不清楚短期饥饿是否影响肠道炎症水平和屏障功能、是否被迷走感觉神经所感应。本研究旨在揭示禁食后肠道炎症水平和肠道屏障变化,以及提高食欲的潜在机制,并提供理论基础和试验依据。
1. 材料与方法
1.1 试验动物与试验设计
选用20只1日龄初生黄羽肉鸡[25-28](购于广东省清远市凤翔麻鸡发展有限公司生产基地),试验前称体质量并排序,随后按配对随机设计的原则将体质量相近的小鼠分为2组:对照组和禁食组,每组10只黄羽肉鸡,正常饲喂饲料至5日龄并采样。采样前12 h,禁食组禁食,对照组正常采食。禁食12 h后收集小肠肠道内容物检测H2O2水平,采集黄羽肉鸡结状神经节(Nodose ganglia,NG),检测炎症和食欲相关受体的表达;采集十二指肠、空肠和回肠及其肠道黏膜,检测黄羽肉鸡肠道形态、闭锁小带蛋白−1 (Zonula,ZO-1)、闭合蛋白 (Occludens-1,OCC)、紧密连接蛋白(Claudin-1) 以及炎症因子的表达。
1.2 测定指标与方法
1.2.1 小肠肠道内容物
分离小肠,区分十二指肠、空肠和回肠,取部分肠道轻轻挤压,将内容物收集于 2 mL 离心管中,使用过氧化氢测定试剂盒(A064-1-1,南京建成生物工程研究所)检测H2O2水平。
1.2.2 扫描电子显微镜(SEM)
取一段1 cm长的空肠,剪开后平铺,用生理盐水轻轻清洗内容物,而后修剪为5 mm边长的正方形放于保存液中,于4 ℃条件下保存。而后脱水、干燥,进行电镜扫描。
1.2.3 苏木精−伊红(HE) 染色
小肠分离后剪取约 3 cm 空肠中段放于 40 g/L 的多聚甲醛中固定,按照常规方法制作石蜡切片,HE染色,光学显微镜下拍照,然后用Image软件测取肠道绒毛长度(lv)和隐窝深度(dc),每个切片取 3~5 个视野,取其平均值计算绒毛长度与隐窝深度比值(lv/dc)。
1.2.4 小肠黏膜及 NG 的 RNA 提取、逆转录和荧光定量PCR (q-PCR)
小肠黏膜及NG总 RNA 使用 RNA 提取试剂盒(R4130-02,广州美基生物科技有限公司)和 TRIzol 试剂提取。1 g 总 RNA 按试剂盒说明书用 4× Reverse Transcription Master Mix(EZB-RT2GQ,美国 EZBioscience 生物技术有限公司)逆转录成 cDNA。引物序列见表1,按照2× SYBR Green qPCR Master Mix(A0012-R2,美国 EZBioscience 生物技术有限公司)说明书配制反应体系:10 μL 的体系中含有 5 μL 2× Color SYBR Green qPCR Master Mix、3.6 μL dd H2O、1 μL cDNA、0.4 μL 引物工作液;使用 Applied Biosystems QuantStudio 3 实时 PCR 系统并按照以下程序反应:95 ℃预热 5 min;95 ℃ 10 s,60 ℃ 30 s,循环 40 次。根据对照组 β-actin mRNA 表达进行归一化处理[15]。
表 1 实时荧光定量PCR所用引物Table 1. Primers used for quantitative real-time PCR基因
Gene上游引物序列(5′→3′)
Forward primer sequence下游引物序列(5′→3′)
Reverse primer sequence序列号
Accession numberβ-actin CTGTGCCCATCTATGAAGGCTA ATTTCTCTCTCGGCTGTGGTG L08165 AgRP CTCTTCCCAGGCCAGACTTG GCAGAAGGCGTTGAAGAACC XM_046925680.1 CCKAR AGCTCTTCTGCCAACCTGAT GTGTAGGACAGCAGGTGGAT NM_001081501.2 Claudin-1 TGGAGGATGACCAGGTGAAG TGTGAAAGGGTCATAGAAGG NM_001013611.2 CART CGAGAGAAGGAGCTGATCGA AGAAAGGAGTTGCACGAGGT XM_046937244.1 FFAR2 GCACTCTCTTTATGGCTGCC GGATTCCCTGGTCTTGGTCA XM_040693461.2 IL-1 CCTCCTCCAGCCAGAAAGTG CGGTAGAAGATGAAGCGGGT XM_015297469.3 IL-4 CCCCAGGTGTAGGCTCTAGT ACTCTGTCATTGCTGCTCCC XM_040683457.2 IL-6 ACCCGAGCTCTTTGGTGATG CGTGCCCTCTGTTTGTACCT XM_025143427.3 IL-10 GCTGCCAAGCCCTGTT CCTCAAACTTCACCCTCA NM_001004414.4 GHSR ATTAGTGCTGGCCCCATCTT CGGACCGATGTTCTTCCTCT XM_046923539.1 MC4R AGGGGTCATCATCACATGCA GATGGCCCCTTTCATGTTGG NM_001031514.2 NPY GTGCTGACTTTCGCCTTGTC ATCTCTGCCTGGTGATGAGG NM_205473.2 Occludin TGGAGGAGTGGGTGAAGAAC ATCCTTCCCCTTCTCCTCCT XM_046904540.1 POMC AGAGGAAGGCGAGGAGGAAA GTAGGCGCTTTTGACGATGG XM_046914234.1 TLR-4 GGCTCAACCTCACGTTGGTA AGTCCGTTCTGAAATCCCGT NM_001030693.2 TNF-α TTCTATGACCGCCCAGTT CAGAGCATCCAACGCAAAA XM_046920820.1 NPY2R GGCCATCATCTCCTATGCCT GGAAGCCAACTGACAGCAAA NM_001398092.1 ZO-1 TCATCCTTACCGCCGCATAT GTTGACTGCTCGTACTCCCT XM_046925214.1 1.3 数据统计与分析
所有数据均以平均值±标准误差(Mean±SE)表示。用GraphPad Prism 8.0 软件进行统计分析。采用 t 检验对2组均值进行差异显著性分析。
2. 结果与分析
2.1 禁食后下丘脑内食欲肽相关受体表达变化
通过 q-PCR 检测下丘脑内食欲肽相关基因表达,结果发现,与对照组相比,雏鸡禁食12 h后促采食食欲肽基因AgRP (P<0.05)和 NPY (P<0.01)的 mRNA 相对表达量均显著上调(图1),提示雏鸡饥饿模型构建成功。
2.2 禁食对黄羽肉鸡空肠肠道形态的影响
空肠肠绒毛电镜扫描及分析结果如图2A、3A、3B 所示,观察发现雏鸡禁食12 h 后,同对照组相比空肠肠绒毛表面更加完整,单位面积内绒毛总数更多、受损更少并且排列更加整齐。空肠 HE 染色及分析结果如图2B、3C、3D 所示,与正常采食的雏鸡相比,禁食后雏鸡的隐窝深度和lv/dc均无明显变化,但是对照组绒毛有明显损伤,而禁食组绒毛排列整齐、长度更长。
![图 3 黄羽肉鸡禁食12 h后空肠肠道绒毛形态变化的电镜扫描结果(A、B)和HE 染色结果(C、D)统计]() 图 3 黄羽肉鸡禁食12 h后空肠肠道绒毛形态变化的电镜扫描结果(A、B)和HE 染色结果(C、D)统计Ⅰ:对照组,Ⅱ:禁食组;“*”和“**”分别表示差异达到 0.05和0.01的显著水平(t检验)Figure 3. Statistics of the scanning electron microscopy results (A, B) and HE staining results (C, D) for the morphological changes of jejunum intestinal villi of yellow-feathered broilers after fasting for 12 hⅠ: Control, Ⅱ: Fasting group; “*” and “**” indicate that the difference reaches 0.05 and 0.01 significance levels respectively (t test)
图 3 黄羽肉鸡禁食12 h后空肠肠道绒毛形态变化的电镜扫描结果(A、B)和HE 染色结果(C、D)统计Ⅰ:对照组,Ⅱ:禁食组;“*”和“**”分别表示差异达到 0.05和0.01的显著水平(t检验)Figure 3. Statistics of the scanning electron microscopy results (A, B) and HE staining results (C, D) for the morphological changes of jejunum intestinal villi of yellow-feathered broilers after fasting for 12 hⅠ: Control, Ⅱ: Fasting group; “*” and “**” indicate that the difference reaches 0.05 and 0.01 significance levels respectively (t test)2.3 禁食对黄羽肉鸡肠道屏障的影响
由图4 可知,与对照组相比,禁食12 h后雏鸡小肠黏膜中紧密蛋白标志性基因ZO-1和Occludin mRNA的相对表达量均显著上调(P<0.05),在十二指肠中,Claudin-1 的mRNA相对表达量也显著上调(P<0.05)。
![图 4 黄羽肉鸡禁食12 h后小肠肠道黏膜紧密蛋白的mRNA相对表达量变化]() 图 4 黄羽肉鸡禁食12 h后小肠肠道黏膜紧密蛋白的mRNA相对表达量变化“*”和“**”分别表示差异达到 0.05和0.01的显著水平(t检验)Figure 4. mRNA relative expression changes of intestinal mucosal compact protein in small intestine of yellow-feathered broilers after 12 h fasting“*” and “**” indicate that the difference reaches 0.05 and 0.01 significance levels respectively (t test)
图 4 黄羽肉鸡禁食12 h后小肠肠道黏膜紧密蛋白的mRNA相对表达量变化“*”和“**”分别表示差异达到 0.05和0.01的显著水平(t检验)Figure 4. mRNA relative expression changes of intestinal mucosal compact protein in small intestine of yellow-feathered broilers after 12 h fasting“*” and “**” indicate that the difference reaches 0.05 and 0.01 significance levels respectively (t test)2.4 禁食对黄羽肉鸡肠道炎症水平的影响
由图5可知,黄羽肉鸡禁食12 h后,与对照组相比,十二指肠、空肠和回肠黏膜上炎症因子IL-1、IL-6和TNF-α的 mRNA表达量无明显变化,但是空肠黏膜抗炎因子IL-4和IL-10的 mRNA表达量均有显著升高(P<0.01)。并且空肠和回肠内容物中H2O2浓度均有不同程度的增加(图3 D )。
![图 5 黄羽肉鸡禁食12 h后小肠炎症因子mRNA相对表达量及H2O2浓度变化]() 图 5 黄羽肉鸡禁食12 h后小肠炎症因子mRNA相对表达量及H2O2浓度变化图D中,DU:十二指肠,Anterior JE:空肠前段,Middle JE:空肠中段,Posterior JE:空肠后段,Anterior IL:回肠前段,Posterior IL:回肠后段;“*”和“**”分别表示差异达到 0.05和0.01的显著水平(t检验)Figure 5. Changes in mRNA relative expressions of intestinal inflammatory factors and H2O2 concentrations in yellow-feathered broilers after 12 h of fastingIn figure D, DU: Duodenum, Anterior JE: Anterior jejunum, Middle JE: Middle jejunum, Posterior JE: Posterior jejunum, Anterior IL: Anterior ileum , Posterior IL: Posterior ileum; “*” and “**” indicate that the difference reaches 0.05 and 0.01 significance levels respectively (t test)
图 5 黄羽肉鸡禁食12 h后小肠炎症因子mRNA相对表达量及H2O2浓度变化图D中,DU:十二指肠,Anterior JE:空肠前段,Middle JE:空肠中段,Posterior JE:空肠后段,Anterior IL:回肠前段,Posterior IL:回肠后段;“*”和“**”分别表示差异达到 0.05和0.01的显著水平(t检验)Figure 5. Changes in mRNA relative expressions of intestinal inflammatory factors and H2O2 concentrations in yellow-feathered broilers after 12 h of fastingIn figure D, DU: Duodenum, Anterior JE: Anterior jejunum, Middle JE: Middle jejunum, Posterior JE: Posterior jejunum, Anterior IL: Anterior ileum , Posterior IL: Posterior ileum; “*” and “**” indicate that the difference reaches 0.05 and 0.01 significance levels respectively (t test)2.5 禁食后雏鸡NG内受体表达的变化
由图6A 可知,与对照组相比,雏鸡禁食12 h后 NG 内肠道炎症因子IL-4的受体基因IL-4R的mRNA相对表达量显著上调(P<0.01)。由图6B 可知,禁食组雏鸡NG内食欲相关受体基因的mRNA相对表达量有所增加,其中FFAR2和神经肽2受体(NPY2R)表达量增加显著(P<0.01)。
![图 6 黄羽肉鸡禁食12 h后结状神经节内炎症(A)与食欲(B)相关受体mRNA相对表达量]() 图 6 黄羽肉鸡禁食12 h后结状神经节内炎症(A)与食欲(B)相关受体mRNA相对表达量“**”表示差异达到0.01的显著水平(t检验)Figure 6. mRNA relative expression of inflammation-related (A) and orexin-related (B) receptors in nodose ganglia of yellow-feathered broilers after 12 h of fasting“**” indicates that the difference reaches 0.01 significance level (t test)
图 6 黄羽肉鸡禁食12 h后结状神经节内炎症(A)与食欲(B)相关受体mRNA相对表达量“**”表示差异达到0.01的显著水平(t检验)Figure 6. mRNA relative expression of inflammation-related (A) and orexin-related (B) receptors in nodose ganglia of yellow-feathered broilers after 12 h of fasting“**” indicates that the difference reaches 0.01 significance level (t test)3. 讨论与结论
已有研究发现,特异性激活下丘脑弓状核AgRP神经元显著提高动物采食量[29],诱导肥胖发生[30],而消除AgRP神经元则会导致厌食症[31]。因此,本研究首先检测了下丘脑弓状核食欲肽表达变化,结果发现短期禁食后黄羽肉鸡下丘脑 AgRP/NPY表达显著上调(P < 0.05),而POMC有下降趋势(P = 0.07),提示黄羽肉鸡饥饿模型构建成功。
肠道健康对机体维持高食欲具有重要作用,而肠道炎症则会影响肠道代谢水平、破坏微生物平衡[32]以及肠道屏障的完整性[33],甚至会影响中枢神经系统中神经肽的分泌,大量研究发现间歇性饥饿有助于维持肠道及肠道屏障的完整性[21-22]。据报道,胃肠道中上皮基质和微生物群落共调控生成活性氧,导致H2O2形成;而H2O2是维持正常细胞稳态和生理功能所必需的第二信使[23]。本试验通过检测小肠不同肠段内容物的H2O2浓度发现,短期禁食导致禁食组空肠和回肠内容物中H2O2浓度均有不同程度的增加,推测饥饿状态下肠道可能通过生成适量H2O2维持肠道稳定。为进一步验证这一假设,我们通过电镜扫描、HE染色以及q-PCR结果发现,短期禁食并未对肠道形态造成损伤,且由于缺少食物影响,肠道绒毛排列更加紧凑整齐。我们推测,机体短期禁食后尚未引发肠道疾病,并且在肠道饥饿状态下,因肠道营养物质缺乏,机体可能出于自我保护机制防止肠道毒素等有害因子进入机体,从而紧密连接增强,即肠道物理屏障增强,且抗炎因子的表达增加,降低空肠损伤比例,避免肠道受损,以抵抗禁食给机体带来的不良影响,维持肠道正常的生理功能,这对维持较高食欲至关重要。
大量研究报道,肠道食欲调控信号不仅可以通过血液信号被中枢所识别,还可以被肠道迷走感觉传入神经元直接感应,经肠−脑轴最终将信号投递至食欲调控中枢[10-12]。本试验结果发现,与对照组相比,雏鸡禁食12 h后结状神经节内IL-4受体基因的mRNA相对表达量显著上调,和肠道黏膜抗炎因子表达变化相对应;提示机体在饥饿状态下,可能通过提高肠道抗炎能力以及增强物理屏障来抵抗由禁食所导致的轻微炎症,维持肠道健康。
此外,结状神经节内食欲相关受体基因的mRNA相对表达量有所增加,其中FFAR2和NPY2R表达量增加显著(P < 0.01),推测黄羽肉鸡饥饿后由于AgRP和NPY表达量增加[4],并且FFAR2和NPY2R表达增加,二者将肠道饥饿信号传递至中枢神经系统,提高动物食欲进而促进采食量增加。
综上所述,饥饿可引起肠道抗炎因子水平升高,并维持肠道屏障完整性,同时促进迷走感觉神经末梢抗炎因子受体表达,最终引起食欲增强。
-
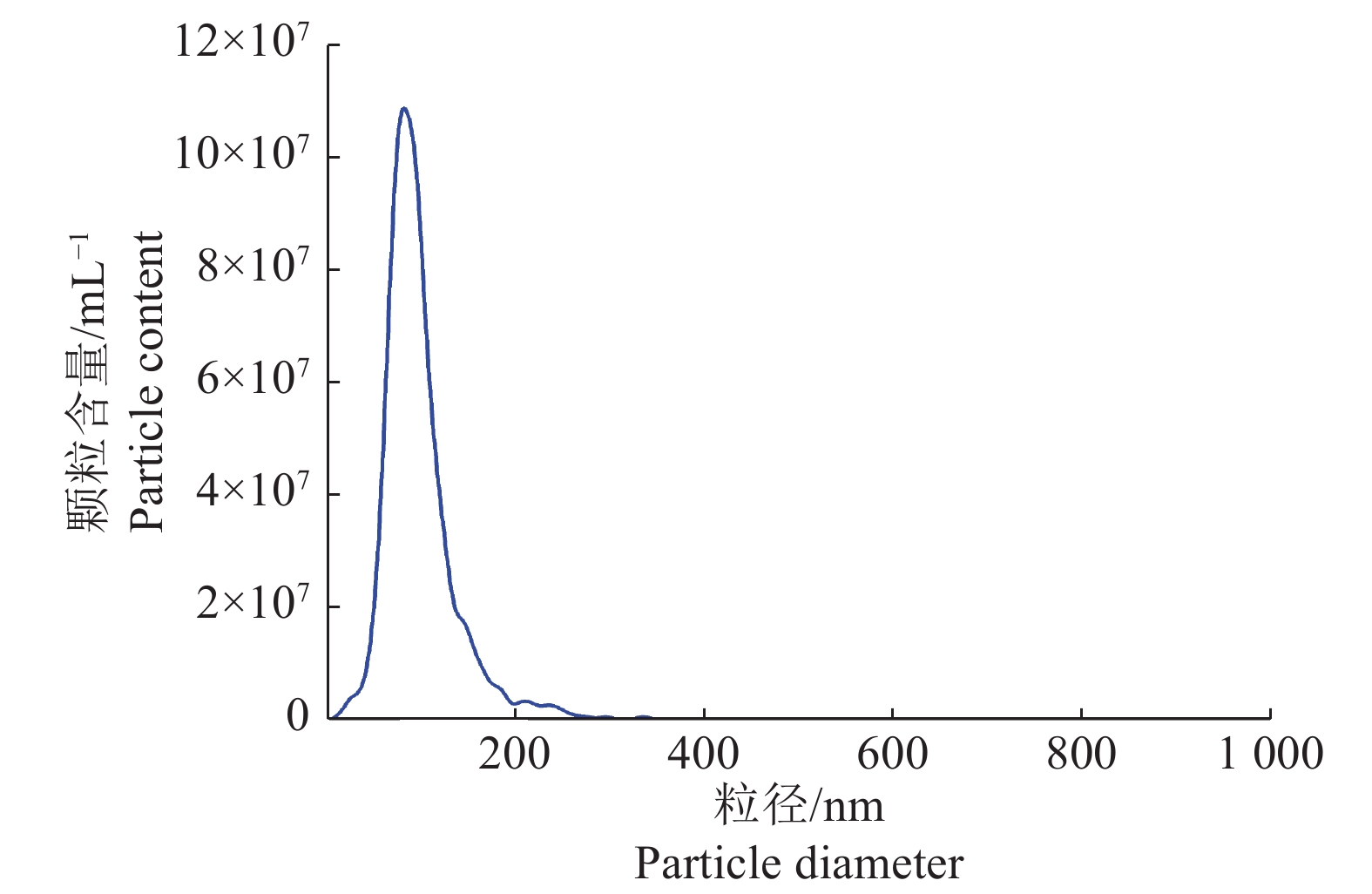
图 2 精浆外泌体粒径分析
精浆外泌体的平均粒径为96.9 nm;粒径主峰为85.2 nm;主峰占检测所有峰的比例为94.9%;颗粒含量为3.4×107 mL−1
Figure 2. Particle size analysis of seminal plasma exosomes
The average particle diameter of seminal plasma exosomes was 96.9 nm; The particle diameter of main peak was 85.2 nm; The main peak accounted for 94.9% of all the detected peaks; The particle content was 3.4×107 mL−1
表 1 精液质量基本信息
Table 1 Basic information of semen quality
公猪编号 Boar number 精子活力/% Sperm vitality 精子畸形率/% Semen malformation rate 精子密度/ mL−1Semen density 1 95 3 4.18×108 2 97 1 3.68×108 3 96 2 4.74×108 4 97 2 3.87×108 5 95 5 5.22×108 表 2 精浆外泌体中标志性miRNA
Table 2 Representative miRNAs in seminal plasma exosomes
miRNA 长度/bp Length 序列(5′→3′) Sequence TPM ssc-let-7c 22 UGAGGUAGUAGGUUGUAUGGUU 851579.7906 ssc-let-7a 22 UGAGGUAGUAGGUUGUAUAGUU 655084.4766 ssc-let-7f-5p 22 UGAGGUAGUAGAUUGUAUAGUU 513356.5287 ssc-let-7e 22 UGAGGUAGGAGGUUGUAUAGUU 426156.8525 ssc-miR-10a-5p 22 UACCCUGUAGAUCCGAAUUUGU 181651.3508 ssc-miR-10b 22 UACCCUGUAGAACCGAAUUUGU 181595.6635 ssc-miR-141 21 UAACACUGUCUGGUAAAGAUG 163842.7352 ssc-miR-21-5p 22 UAGCUUAUCAGACUGAUGUUGA 125987.7562 ssc-miR-125b 22 UCCCUGAGACCCUAACUUGUGA 118310.9973 ssc-miR-200b 23 UAAUACUGCCUGGUAAUGAUGAC 99945.7705 ssc-miR-16 22 UAGCAGCACGUAAAUAUUGGCG 96309.6121 ssc-miR-30a-5p 22 UGUAAACAUCCUCGACUGGAAG 93885.9728 ssc-miR-30d 24 UGUAAACAUCCCCGACUGGAAGCU 74152.9510 ssc-miR-148a-3p 22 UCAGUGCACUACAGAACUUUGU 73369.8562 ssc-miR-191 23 CAACGGAAUCCCAAAAGCAGCUG 65102.6570 ssc-miR-26a 22 UUCAAGUAAUCCAGGAUAGGCU 59547.6681 ssc-miR-26b-5p 22 UUCAAGUAAUUCAGGAUAGGUU 53532.4383 ssc-miR-125a 23 UCCCUGAGACCCUUUAACCUGUG 51427.2644 ssc-let-7i-5p 19 UGAGGUAGUAGUUUGUGCU 50378.3688 ssc-miR-29c 22 UAGCACCAUUUGAAAUCGGUUA 44726.2430 -
[1] SHAO H, IM H, CASTRO C M, et al. New technologies for analysis of extracellular vesicles[J]. Chemical Reviews, 2018, 118(4): 1917-1950. doi: 10.1021/acs.chemrev.7b00534
[2] SUN J, ZHAO Y, HE J, et al. Small RNA expression patterns in seminal plasma exosomes isolated from semen containing spermatozoa with cytoplasmic droplets versus regular exosomes in boar semen[J]. Theriogenology, 2021, 176: 233-243. doi: 10.1016/j.theriogenology.2021.09.031
[3] 杜冠潮, 王福, 张继伟, 等. 精浆外泌体在精子成熟过程中的调节机制研究进展[J]. 山东医药, 2020, 60(36): 105-107. doi: 10.3969/j.issn.1002-266X.2020.36.028 [4] THERY C, OSTROWSKI M, SEGURA E. Membrane vesicles as conveyors of immune responses[J]. Nature Reviews Immunology, 2009, 9(8): 581-593. doi: 10.1038/nri2567
[5] SAINT-DIZIER M, MAHE C, REYNAUD K, et al. Sperm interactions with the female reproductive tract: A key for successful fertilization in mammals[J]. Molecular and Cellular Endocrinology, 2020, 516: 110956. doi: 10.1016/j.mce.2020.110956
[6] 秦佳丽, 孙敬帅, 曹婷婷, 等. 精浆外泌体的转运机制及生物学功能研究进展[J]. 中国畜牧杂志, 2022, 58(2): 52-58. [7] GUO H, CHANG Z, ZHANG Z, et al. Extracellular ATPs produced in seminal plasma exosomes regulate boar sperm motility and mitochondrial metabolism[J]. Theriogenology, 2019, 139: 113-120. doi: 10.1016/j.theriogenology.2019.08.003
[8] 杨秀芹, 万洪宇, 王金奎, 等. 猪miR-101表达特性分析[J]. 东北农业大学学报, 2016, 47(6): 74-80. [9] 曲波, 甄贞, 仇有文, 等. 基于生物信息学方法挖掘奶山羊miRNAs研究[J]. 东北农业大学学报, 2015, 46(1): 86-93. doi: 10.3969/j.issn.1005-9369.2015.01.014 [10] TWENTER H, KLOHONATZ K, DAVIS K, et al. Transfer of MicroRNAs from epididymal epithelium to equine spermatozoa[J]. Journal of Equine Veterinary Science, 2020, 87: 102841. doi: 10.1016/j.jevs.2019.102841
[11] FOSHAY K M, GALLICANO G I. miR-17 family miRNAs are expressed during early mammalian development and regulate stem cell differentiation[J]. Developmental Biology, 2009, 326(2): 431-443. doi: 10.1016/j.ydbio.2008.11.016
[12] BOUHALLIER F, ALLIOLI N, LAVIAL F, et al. Role of miR-34c microRNA in the late steps of spermatogenesis[J]. RNA, 2010, 16(4): 720-731. doi: 10.1261/rna.1963810
[13] 薛林涛, 黄悦悦, 施文. 精浆外泌体在精子发生与功能调控中的研究进展[J]. 右江医学, 2021, 49(9): 706-709. [14] 吴志胜, 陈慧芳, 刘俊杰, 等. 长白猪精浆外泌体miRNAs的鉴定与功能分析[J]. 农业生物技术学报, 2021, 29(2): 279-287. [15] 白绪祥, 韩帅琪, 胡建宏. 猪精液常温保存研究进展[J]. 畜牧兽医杂志, 2021, 40(1): 28-30. [16] 舒密. 外泌体miRNAs在结直肠癌中的研究进展[J]. 医学信息, 2021, 34(15): 15-18. [17] 卞玉莹. 精浆外泌体miRNA作为男性不育症新型分子标志物的临床研究[D]. 镇江: 江苏大学, 2020. [18] MCGRAW L A, SUAREZ S S, WOLFNER M F. On a matter of seminal importance[J]. BioEssays, 2015, 37(2): 142-147. doi: 10.1002/bies.201400117
[19] TEOW S, LIEW K, KHOO A S, et al. Pathogenic role of exosomes in Epstein-Barr virus (EBV)-associated cancers[J]. International Journal of Biological Sciences, 2017, 13(10): 1276-1286. doi: 10.7150/ijbs.19531
[20] 陈慧芳, 杨镁楹, 吴志胜, 等. 外泌体miRNA在配子发育和受精中的调控作用[J]. 中国畜牧杂志, 2021, 57(1): 17-24. doi: 10.19556/j.0258-7033.20200304-01 [21] SANTONOCITO M, VENTO M, GUGLIELMINO M R, et al. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation[J]. Fertility and Sterility, 2014, 102(6): 1751-1761. doi: 10.1016/j.fertnstert.2014.08.005
[22] ROUSH S, SLACK F J. The let-7 family of microRNAs[J]. Trends in Cell Biology, 2008, 18(10): 505-516. doi: 10.1016/j.tcb.2008.07.007
[23] LUO Z, DAI X, RAN X, et al. Identification and profile of microRNAs in Xiang pig testes in four different ages detected by Solexa sequencing[J]. Theriogenology, 2018, 117: 61-71. doi: 10.1016/j.theriogenology.2017.06.023
[24] CURRY E, SAFRANSKI T J, PRATT S L. Differential expression of porcine sperm microRNAs and their association with sperm morphology and motility[J]. Theriogenology, 2011, 76(8): 1532-1539. doi: 10.1016/j.theriogenology.2011.06.025
[25] LUO M, HAO L, HU F, et al. MicroRNA profiles and potential regulatory pattern during the early stage of spermatogenesis in mice[J]. Science China Life Sciences, 2015, 58(5): 442-450. doi: 10.1007/s11427-014-4737-8
[26] WU W, HU Z, QIN Y, et al. Seminal plasma microRNAs: Potential biomarkers for spermatogenesis status[J]. Molecular Human Reproduction, 2012, 18(10): 489-497. doi: 10.1093/molehr/gas022
[27] HOSSEINI S, HOSSEINI S, SALEHI M. Upregulation of Toll-like receptor 4 through anti-miR-Let-7a enhances blastocyst attachment to endometrial cells in mice[J]. Journal of Cellular Physiology, 2020, 235(12): 9752-9762. doi: 10.1002/jcp.29787
[28] BISSONNETTE N, LÉVESQUE-SERGERIE J, THIBAULT C, et al. Spermatozoal transcriptome profiling for bull sperm motility: A potential tool to evaluate semen quality[J]. Reproduction (Cambridge, England), 2009, 138(1): 65-80. doi: 10.1530/REP-08-0503
[29] WANG W, LIANG K, CHANG Y, et al. miR-26a is Involved in glycometabolism and affects boar sperm viability by targeting PDHX[J]. Cells, 2020, 9(1): 146. doi: 10.3390/cells9010146
[30] 范宇. 睾酮缺乏诱导miR-26a-5p和let-7g-5p作为信号递质靶向作用于PTEN和PMAIP1调控公猪精子细胞凋亡[D]. 雅安: 四川农业大学, 2018. [31] GODIA M, CASTELLÓ A, ROCCO M, et al. Identification of circular RNAs in porcine sperm and evaluation of their relation to sperm motility[J]. Scientific Reports, 2020, 10(1): 1-11. doi: 10.1038/s41598-019-56847-4
[32] GONZALEZ-GONZALEZ E, LOPEZ-CASAS P P, DEL MAZO J. Gene silencing by RNAi in mouse Sertoli cells[J]. Reproductive Biology and Endocrinology, 2008, 6: 29. doi: 10.1186/1477-7827-6-29
[33] KOTAJA N, BHATTACHARYYA S N, JASKIEWICZ L, et al. The chromatoid body of male germ cells: Similarity with processing bodies and presence of Dicer and microRNA pathway components[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(8): 2647-2652. doi: 10.1073/pnas.0509333103
[34] HUNTER M P, ISMAIL N, ZHANG X, et al. Detection of microRNA expression in human peripheral blood microvesicles[J]. PLoS One, 2008, 3(11): e3694. doi: 10.1371/journal.pone.0003694
[35] 梅星星, 李小勇, 吴际. 一组miRNAs在睾丸发育中的表达及miR-125a对精原干细胞发育的调节作用[J]. 上海交通大学学报(医学版), 2015, 35(5): 625-630. [36] LI J, LIU X, HU X, et al. MicroRNA-10b regulates the renewal of spermatogonial stem cells through Kruppel-like factor 4[J]. Cell Biochemistry and Function, 2017, 35(3): 184-191. doi: 10.1002/cbf.3263
[37] 王道光. p53蛋白介导的细胞DNA损伤响应的动力学机制研究[D]. 南京: 南京大学, 2017. [38] SANTO G D, FRASCA M, BERTOLI G, et al. Identification of key miRNAs in prostate cancer progression based on miRNA-mRNA network construction[J]. Computational and Structural Biotechnology Journal, 2022, 20: 864-873. doi: 10.1016/j.csbj.2022.02.002
[39] RAUHALA H E, JALAVA S E, ISOTALO J, et al. miR-193b is an epigenetically regulated putative tumor suppressor in prostate cancer[J]. International Journal of Cancer, 2010, 127(6): 1363-1372. doi: 10.1002/ijc.25162
[40] MERKULOVA M, PĂUNESCU T G, AZROYAN A, et al. Mapping the H+ (V)-ATPase interactome: Identification of proteins involved in trafficking, folding, assembly and phosphorylation[J]. Scientific Reports, 2015, 5: 14827. doi: 10.1038/srep14827
[41] SILVA J V, SANTIAGO J, SOUSA M, et al. New evidences of ubiquitin-proteasome system activity in human sperm[J]. Biochimica et Biophysica Acta-Molecular Cell Research, 2021, 1868(3): 118932. doi: 10.1016/j.bbamcr.2020.118932
[42] IJIRI T W, MERDIUSHEV T, CAO W, et al. Identification and validation of mouse sperm proteins correlated with epididymal maturation[J]. Proteomics, 2011, 11(20): 4047-4062. doi: 10.1002/pmic.201100075



 下载:
下载: