Ablation effect of busulfan on pig endogenous spermatogonial stem cells and transplantation of exogenous spermatogonial stem cells
-
摘要:目的
研究猪睾丸组织注射白消安消融猪内源精原干细胞(Spermatogonial stem cell,SSC)的效果,以及猪SSC同种异体移植后对内源性SSC消融受体生殖能力恢复的影响。
方法采用3 mg/kg剂量的白消安对9头6周龄大白公猪进行睾丸注射,另外3头注射2 mL 二甲基亚砜作为对照。3周后,对试验组公猪以相同剂量进行第2次睾丸注射。第2次注射3周后,采集试验组和对照组公猪睾丸进行相关检测,评估内源性SSC消融情况。第2次白消安注射1个月后,以两步酶消化法处理5~7日龄大白仔猪睾丸分离得到睾丸单细胞悬液,并用明胶差速贴壁法进行纯化,纯化后以免疫荧光和流式细胞术分析SSC的纯度。异体移植SSC 4个月后,用微卫星标记检测受体公猪精液以及睾丸组织中供体来源SSC的存在。
结果以3 mg/kg剂量的白消安处理大白公猪2次后,睾丸组织苏木精−伊红染色以及免疫组织化学染色结果显示,试验组睾丸曲细精管中各级生精细胞消融但其支持细胞结构完好,可以支持外源性SSC的定植及发育。免疫荧光以及流式细胞术结果表明,分离得到的睾丸单细胞悬液经纯化后UCHL-1阳性细胞占比由差速贴壁前的16.3%提高到了50.8%。苏木精−伊红染色以及免疫组织化学染色结果显示,猪SSC移植4个月后,移植组睾丸组织生精细胞恢复,在受体睾丸曲细精管基底膜上可检测到UCHL-1阳性SSC。受体睾丸组织的微卫星标记分析显示了供体SSC的存在,表明移植进入受体睾丸中的供体SSC可以在受体睾丸中定植存活超过4个月;精液微卫星标记未检测到供体来源的精子。
结论以3 mg/kg剂量的白消安注射公猪睾丸能有效消融内源性SSC,可以用来制备SSC移植的受体猪。两步酶消化及明胶差速贴壁法可成功分离纯化猪SSC。猪SSC经同种异体移植后可以在受体睾丸中定植存活超过4个月。
Abstract:ObjectiveTo study the effect of intratubular injection of busulfan on ablation of pig endogenous spermatogonial stem cells (SSCs) and allotransplantation of SSCs on the recovery of the reproductive ability of the recipient after ablation of endogenous SSCs.
MethodNine 6-week-old Large-white boars were injected through seminiferous tubules with busulfan at a dosage of 3 mg/kg, and other three pigs were injected with 2 mL DMSO as controls. After three weeks, each boar in the test group was given a second injection at the same dosage. At three weeks after the second injection, testes from test and control groups were collected to evaluate the endogenous SSC ablation conditions. At one month after the second busulfan injection, testicular single-cell suspension was isolated from the testes of 5−7-day-old Large-white piglets by two-step enzyme digestion method and purified by gelatin differential adherence method. After purification, the purity of SSCs was analyzed by immunofluorescence and flow cytometry. At four months after allotransplantation of SSCs, microsatellite marker analysis was used to detect the presence of donor-derived SSCs in recipient boar semen and testicular tissue.
ResultAfter treating the Large-white boar twice with busulfan at a dosage of 3 mg/kg, the results of HE staining and immunohistochemistry staining of testis tissue showed that germ cells at all levels in the seminiferous tubules of the testes in test group were ablated but the structure of sertoli cell was intact, which could support the colonization and development of transplanted exogenous SSC. The results of immunofluorescence and flow cytometry showed that the rate of UCHL-1 positive cells in the isolated testicular single-cell suspension increased from 16.3% before differential adhesion to 50.8% after purification. At four months after allotransplantation of porcine SSC, HE staining and immunohistochemical staining of testicular tissues in the transplanted group showed recovery of germ cell layers compared with the group injected with busulfan but not transplanted with donor cells. UCHL-1 positive SSCs were detected on the basement membrane of seminiferous tubules in recipient testes. Microsatellite marker analysis of recipient testis tissue showed the presence of donor SSCs, suggesting that donor SSCs transplanted into recipient testes could colonize and survive in recipient testes for more than four months. However, microsatellite marker analysis of semen did not detect donor-derived sperms.
ConclusionIntratubular injection of busulfan into boar testes at a dosage of 3 mg/kg can effectively ablate endogenous SSCs and can be used to prepare recipient pigs for SSC transplantation. Two-step enzyme digestion and gelatin differential adhesion method can be used to successfully isolate and purify porcine SSCs. Porcine SSCs can colonize in recipient testes and survive for more than four months after allotransplantation.
-
Keywords:
- pig /
- spermatogonial stem cell /
- busulfan /
- cell transplantation /
- spermatogenesis
-
精原干细胞(Spermatogonial stem cell,SSC)是位于雄性哺乳动物睾丸曲细精管基底膜上的一类成体干细胞,它既可以通过自我更新不断进行增殖以维持自身数量的稳定,又能定向分化成各级生精细胞并产生功能性精子以维持精子发生过程的稳定,从而将遗传信息传递给下一代。精子发生(Spermatogenesis)是一个高度复杂且具有组织协调性的过程,维持着雄性哺乳动物一生中精子的高效产生,而精子发生系统的高效性依赖于SSC的不断增殖与分化[1]。SSC移植(Spermatogonial stem cell transplantation,SSCT),即注入的供体SSC由受体睾丸曲细精管的管腔迁移至基底膜上,定植到SSC生存所需的微环境“niche”中,并经历自我更新以及定向分化进而建立起稳定的供体来源的精子发生的过程[2]。1994年,Brinster团队首次将含有SSC的细胞悬液移植到不育受体小鼠睾丸的曲细精管中,观察到了完整的供体细胞来源的精子发生并获得了成熟的精子,经自然交配获得了供体来源的后代,而且该研究发现在受体小鼠睾丸中移植精原细胞似乎比生殖母细胞更有效。这项技术的突破为生产不同物种的遗传修饰动物提供了一种新方法,并将在生物医学和农业等领域有更多的应用[3-4]。在随后的20多年间,SSCT被应用到猪Sus scrofa[5-8]、牛Bos taurus[9-11]、山羊Capra hircus[12-14]、绵羊Ovis aries[15-18]、食蟹猴Macaca fascicularis[19-20]、恒河猴Macaca mulatta[21-24]、狗Canis lupusfamiliaris[25-26]、树鼩Tupaia belangeris[27]、单峰骆驼Camelus dromedarius[28]等大动物上,不仅在受体睾丸中观察到供体生殖细胞的定植、供体来源的精子发生以及得到了供体来源的猪、恒河猴的体外授精胚胎[23, 29-32],而且利用腹腔镜辅助人工授精获得了供体来源的绵羊后代[17],经自然交配获得了供体来源的山羊后代[12]以及供体来源的树鼩后代[27]。
利用小鼠模型,研究人员开发了3种可行的SSCT方法,即通过显微注射的手段将含SSC的供体细胞悬液引入受体睾丸的曲细精管(Seminiferous tubules)、睾丸中所有曲细精管末端汇聚形成的睾丸网区域(Rete testis)以及从睾丸网伸出并连接到附睾头部的3~6根输出小管(Efferent duct)[3-4, 33]。然而,由于不同物种间睾丸大小和解剖学结构的差异性,这3种移植方法在推广应用到大动物上时需要做适当的改进。目前,在家畜等大动物上应用最广泛的是通过超声介导或外科手术解剖的方式将供体细胞悬液引入到受体睾丸的睾丸网区域进而扩散到整个睾丸的大部分曲细精管中[29-32]。无论采用哪种策略引入供体SSC,要实现定植,SSC都必须从受体睾丸曲细精管内腔迁移至基底膜上支持SSC存活的“niche”,才能重新建立供体SSC来源的精子发生,而内源性SSC的存在会降低供体SSC定植的机率。因此,内源性SSC耗竭的同时保留受体睾丸中支持细胞(Sertoli cell)正常结构的理想受体对于供体SSC的植入至关重要[34]。白消安是一种可以诱导分裂细胞凋亡的烷化剂,由于SSC具有较高的有丝分裂活性,因而对抑制有丝分裂的药物和诱导细胞凋亡的白消安敏感[35-36]。白消安药物处理受体睾丸引起的内源性SSC消融具有剂量依赖性,有利于我们通过调整注射剂量来制备理想的受体,因此白消安被广泛应用于制备适用于SSCT的理想受体[6, 22, 27, 37-38]。
本研究尝试通过对猪睾丸直接进行白消安注射消除内源SSC,探讨一种简明方便且毒副作用较低的SSCT受体制备方案。将分离纯化后的供体仔猪SSC移植到经白消安处理后内源性SSC消融的受体睾丸中,移植后采集受体公猪睾丸组织和精液进行组织学和微卫星标记分析,研究SSC的定植和受体生精能力的恢复情况。
1. 材料与方法
1.1 试验材料
白消安、DMSO均购自Sigma公司;断奶仔猪购自温氏食品集团种猪科技公司水台繁殖场;兽用B型超声诊断仪购自深圳维尔德医疗电子有限公司;一次性使用人体静脉血样采集容器(肝素钠)购自广州和粤生物科技有限公司;5~7日龄大白仔猪睾丸由温氏食品集团种猪科技公司东成试验场提供;DMEM、DMEM/F12、FBS、DPBS、质量分数为0.25%的胰蛋白酶−EDTA、双抗(青链霉素)均为Gibco产品,购自英潍捷基(上海)贸易有限公司;胶原酶Ⅳ、明胶为Millipore产品,购自上海优宁维生物科技股份有限公司;一次性40 μm尼龙细胞筛(BD)购自广州誉维生物科技仪器有限公司;组织和精液DNA提取试剂盒(Tissue DNA Kit D3396、Forensic DNA Kit D3591)购自广州飞扬生物工程有限公司。
1.2 试验方法
1.2.1 注射白消安以消融受体公猪内源性SSC
采用白消安对9头6周龄大白公猪进行睾丸注射,剂量为3 mg/kg;另外3头注射2 mL二甲基亚砜(DMSO)作为对照。白消安注射3周后,对试验组公猪进行第2次相同剂量的白消安睾丸注射。第2次注射3周后,采集试验组和对照组公猪睾丸进行相关检测,评估内源性SSC消融情况。试验期间注意改善公猪饲粮并细心照料。
1.2.2 睾丸组织石蜡切片制作
第2次注射白消安3周后,采集1头试验组和对照组公猪睾丸组织置于体积分数为4%的中性多聚甲醛溶液中固定。固定完成后进行乙醇梯度脱水,即70%(φ)乙醇溶液2.0 h、80%(φ)乙醇溶液2.0 h、95%(φ)乙醇溶液1.5 h、95%(φ)乙醇溶液1.5 h、无水乙醇0.5 h、无水乙醇0.5 h。随后的透明过程不仅可以脱去乙醇,还可以溶解石蜡,有利于石蜡渗入组织为下一步的浸蜡创造条件。透明的时间和过程如下:无水乙醇+二甲苯(体积比为1∶1)轻蘸、二甲苯15 min、二甲苯15 min,第2次时间由第1次二甲苯透明的效果决定,最终使组织呈半透明状。经浸蜡包埋后固定在切片机上,将切片厚度调整为5 μm,连续切片5圈。切出的蜡片放入38 ℃的水浴锅中使其完全舒展开,取载玻片轻轻地捞起蜡片,随后放入电热鼓风干燥箱中60 ℃烘烤2 h即可进行染色。
1.2.3 睾丸组织的苏木精−伊红染色及免疫组织化学处理
睾丸石蜡切片制作完成后先后进行苏木精−伊红(Hematoxylin-eosin,HE)染色和免疫组织化学(Immunohistochemistry,IHC)染色处理。即将石蜡切片依次进行脱蜡复水、苏木精染色、伊红染色、脱水透明、中性树胶封片制作成HE染色切片,随后显微镜镜检,分析并拍照。同样,将睾丸石蜡切片依次进行脱蜡复水、柠檬酸(pH 6.0)抗原修复液进行抗原修复、阻断内源性过氧化物酶、血清封闭、一抗(Ubiquitin carboxy-terminal hydrolase L1,UCHL-1)孵育、二抗孵育、二氨基联苯胺(Diaminobenzidine, DAB)显色、细胞核的苏木精复染制作成IHC切片,最后显微镜镜检,分析并拍照。
1.2.4 白消安处理后猪血常规分析
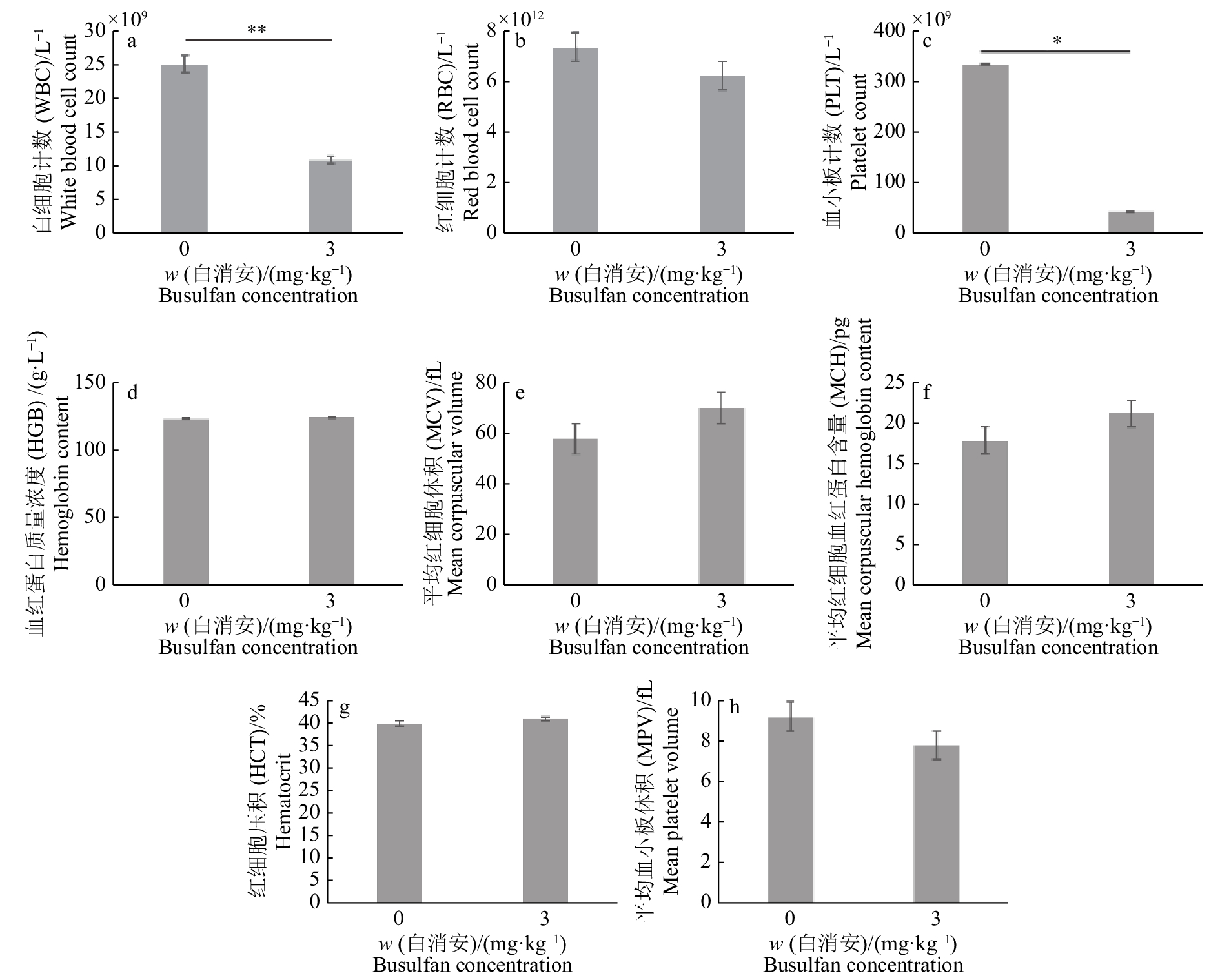
以3 mg/kg剂量白消安注射公猪睾丸6周后,对试验组以及对照组公猪进行前腔静脉采血,采血完成后随即用肝素钠抗凝管低温运输到广东省医学实验动物中心进行血常规检测,对相关指标进行数据分析以评估注射白消安后对公猪造血功能的影响。
1.2.5 猪睾丸生殖细胞的分离
取5~7日龄大白仔猪睾丸,置于75%(φ)乙醇溶液中浸泡5 min,DPBS清洗3次。随后用已高压灭菌的镊子和剪刀去除附睾及其他结缔组织,经DPBS清洗后换新的镊子和剪刀去除睾丸白膜,将暴露的睾丸组织剪碎并转移至15 mL离心管中,加入1 mg/mL的胶原酶Ⅳ,于37 ℃水浴锅中消化15 min,4 014 r/min离心5 min,去上清液;加入质量分数为0.25%的胰蛋白酶−EDTA于 37 ℃水浴锅中消化5~10 min,添加体积分数为10% 的FBS终止胰蛋白酶的消化,4 014 r/min离心5 min,去上清液。DPBS洗3次,细胞沉淀用完全培养基重悬,并用40 μm尼龙细胞筛过滤得到单细胞悬液。两步酶消化过程中添加DNaseⅠ(20 U/mL)以避免睾丸组织出现黏连聚集,期间多次晃动以加快消化。
1.2.6 猪SSC的纯化
将上述得到的睾丸单细胞悬液平铺于经明胶包被的10 cm细胞培养板上,于37 ℃、CO2体积分数为5%的培养箱中培养。睾丸单细胞悬液经差速贴壁2次,每次2 h,最后转移至新的明胶包被的10 cm细胞培养板上过夜培养,第2天收集未贴壁的细胞悬液即为纯化后的SSC。纯化后的SSC经4 014 r/min 离心5 min,去上清液,用预热的DPBS重悬,37 ℃条件下带往猪场进行移植。
1.2.7 猪SSC的鉴定
取纯化后的SSC,4 014 r/min离心5 min,去上清液,用体积分数为4%的多聚甲醛溶液固定30 min;加适量DPBS洗涤3遍,4 014 r/min 离心5 min;加适量免疫染色通透液(Triton X-100),室温通透10 min;弃去通透液,DPBS洗涤3次,加入QuickBlock免疫染色封闭液,室温封闭10 min;弃去封闭液,DPBS洗涤3次,QuickBlock免疫染色一抗稀释液以体积比1∶200稀释UCHL-1抗体,覆盖细胞于4 ℃孵育过夜;DPBS洗涤3次,QuickBlock免疫染色二抗稀释液以体积比1∶500倍稀释的Anti-Mouse IgG(Alexa Flour 488)二抗,覆盖住细胞,避光条件下于37 ℃孵育2 h;DPBS洗涤3次,加入适量的4′,6−二脒基−2−苯基吲哚(4′,6-diamidino-2-phenylindole, DAPI)染色液覆盖住细胞,避光条件下室温放置10 min;DPBS洗涤3次,荧光显微镜下观察并拍照。
1.2.8 流式细胞术分析SSC纯度
取纯化后的SSC,4 014 r/min 离心5 min,去上清液,加体积分数为4%的多聚甲醛溶液固定30 min;固定结束后4 014 r/min离心5 min,去上清液,加入免疫染色通透液(Triton X-100),室温通透10 min;通透结束后4 014 r/min离心5 min,去上清液,加入QuickBlock免疫染色封闭液,室温封闭10 min;弃去封闭液,DPBS洗涤1次,QuickBlock免疫染色一抗稀释液以体积比1∶200稀释UCHL-1抗体,覆盖细胞于室温孵育30 min;DPBS洗涤1次,4 014 r/min离心5 min,加入以体积比1∶500稀释的Anti-Mouse IgG(Alexa Flour 488)二抗,避光条件下室温孵育20 min,DPBS洗涤1次,随后用DPBS重悬细胞,流式细胞仪检测。
1.2.9 猪SSC的同种异体移植
将经白消安处理的受体公猪赶至限位栏中,用保定绳进行保定,耳缘静脉注射麻醉药进行麻醉。待受体公猪麻醉后将其侧放,用干净的湿毛巾清洗猪睾丸部位,涂抹超声造影剂。用便携式B超探头沿睾丸纵轴方向探测出睾丸网的位置,将注射针从睾丸上端缓缓伸入到睾丸网区域,保持注射针的位置并缓缓将SSC悬液推入,每头公猪每侧睾丸注射5~7 mL。注射完成后对公猪注射部位涂抹碘酊消毒,同时改善公猪饲粮并细心照看。
1.2.10 受体公猪调教与精液采集
待移植后受体公猪达7月龄时,进行数次调教以采集精液。即将发情母猪的尿液涂抹在假母台上,引诱受体公猪前往假母台爬跨,待公猪成功爬跨数次后即可进行采精。采集到的精液用配制好的精液稀释液进行稀释,注意稀释过程中两者温度相差不能超过1 ℃,稀释好的精液在20 ℃条件下带回实验室进行下一步试验。对经数次调教仍未能成功采集精液的公猪进行阉割,取睾丸组织或附睾精子进行后续微卫星标记分析。
1.2.11 DNA提取与微卫星标记分析
供、受体公猪基因组DNA、精液DNA提取均采用OMEGA公司的试剂盒。对于采精成功的2头公猪,供、受体基因组DNA以及精液DNA提取完成后送往华大基因进行微卫星标记分析以判断精液DNA的来源;对于未能成功采精的3头公猪,供、受体基因组DNA以及受体睾丸组织DNA提取完成后送往华大基因进行微卫星标记分析以判断受体公猪曲细精管中是否存在供体来源的精子,进而验证猪SSCT是否成功。微卫星标记分析的引物按照国际动物遗传学会和联合国粮农组织公布的《发展中国家农业动物遗传资源管理计划指南》[39]中推荐的微卫星标记的引物序列交由华大基因合成引物并测序。测定的微卫星位点信息见表1。
表 1 微卫星多态性分析引物序列Table 1. Primer sequences for polymorphic microsatellites analysis位点名称
Locus name所在染色体
Chr.引物序列1)(5′→3′)
Primer sequence退火温度/ ℃
Annealing temperature等位基因长度范围/bp
Allele rangeS0155 1 D-TGTTCTCTGTTTCTCCTCTGTTTG
AAAGTGGAAAGAGTCAATGGCTAT55 142~162 S0355 15 D-TCTGGCTCCTACACTCCTTCTTGATG
TTGGGTGGGTGCTGAAAAATAGGA55 244~271 Sw857 14 D-TGAGAGGTCAGTTACAGAAGACC
GATCCTCCTCCAAATCCCAT55 141~159 1)字母“D”表示带荧光标记的引物
1) Letter“D” indicates dye labelled primer1.2.12 统计分析
采用SPSS Statistics 20软件对数据进行统计学分析。2组数据的比较分析采用独立样本t检验。
2. 结果与分析
2.1 睾丸注射白消安后制备内源SSC消融的受体
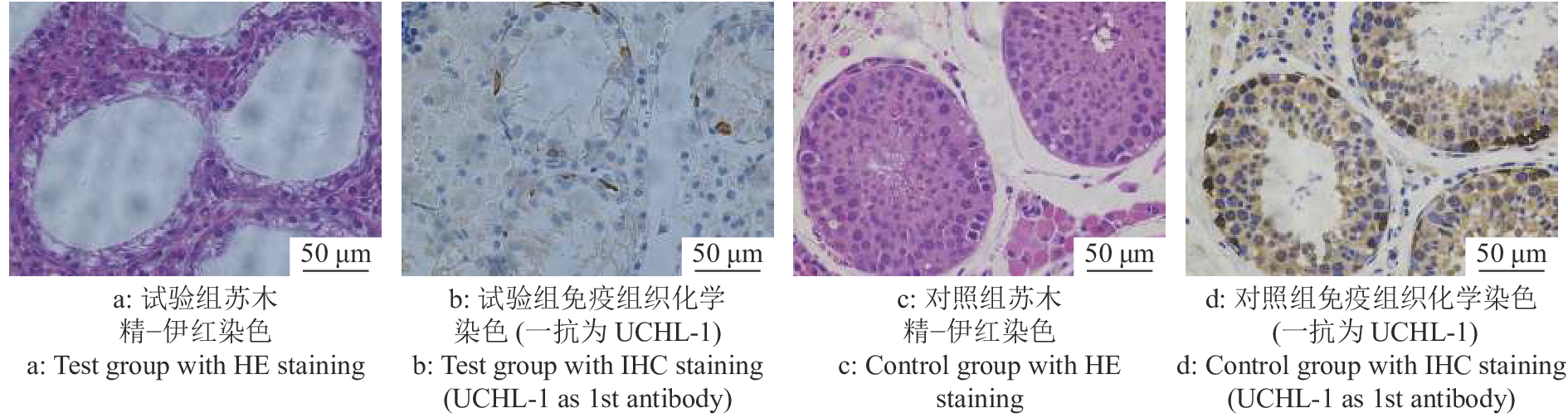
试验组公猪每侧睾丸注射3 mg/kg剂量的白消安6周后,公猪睾丸组织HE染色结果表明,多数曲细精管中的各级生精细胞消融殆尽,呈现出了“唯支持细胞”表型的空洞状曲细精管结构(图1a、1c)。同时,试验组IHC染色结果与对照组相比,曲细精管基底膜上几乎不存在UCHL-1阳性的SSC(图1b、1d),证明了白消安对包括SSC在内的生殖细胞的杀伤作用。在对试验组及对照组公猪白消安处理6周后进行的血常规检测分析显示,试验组公猪血液中的白细胞计数和血小板计数与对照组相比明显下降。其他如红细胞计数、血红蛋白质量浓度、平均红细胞体积、平均红细胞血红蛋白含量、红细胞压积、平均血小板体积等与对照组相比无明显差异且均在正常范围内(图2)。这些结果显示了局部的白消安注射短期内会对造血功能造成一定的影响,但是我们并未观察到猪只的健康状况受到明显影响。并且我们前期的研究结果也显示3 mg/kg白消安进行睾丸注射6个月后试验猪只的血常规已恢复到正常水平(未发表)。综合以上结果表明,3 mg/kg剂量的白消安注射公猪睾丸可以消融曲细精管中包括SSC在内的生殖细胞,给外源SSC的移植提供了可定植的微环境。
2.2 猪SSC的分离与纯化
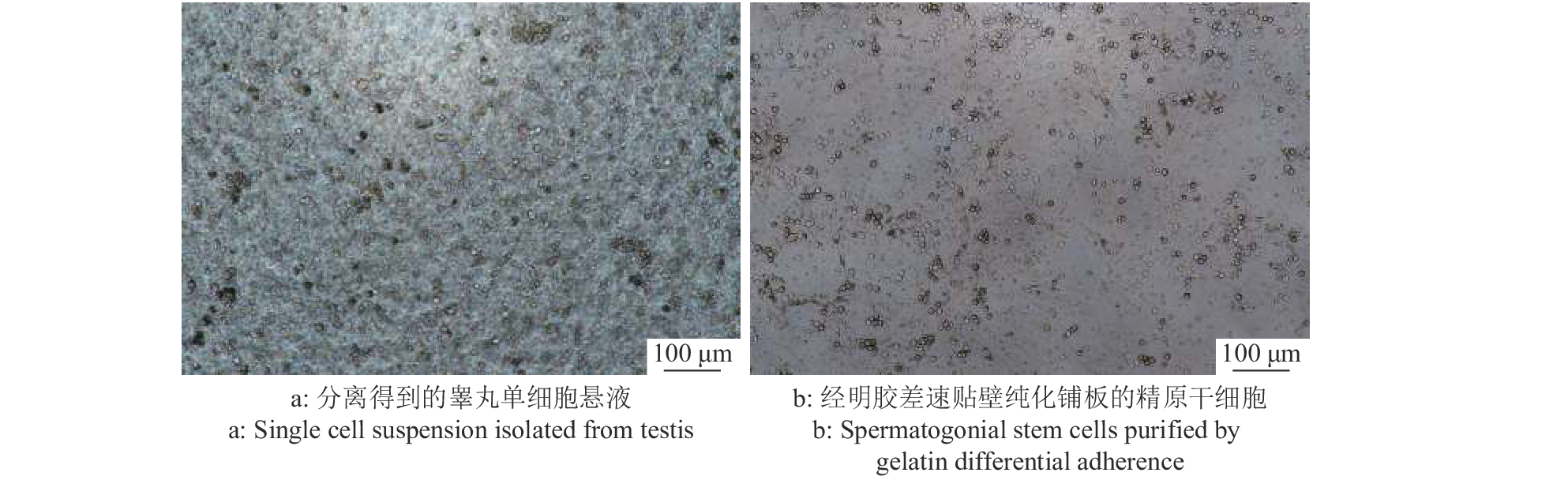
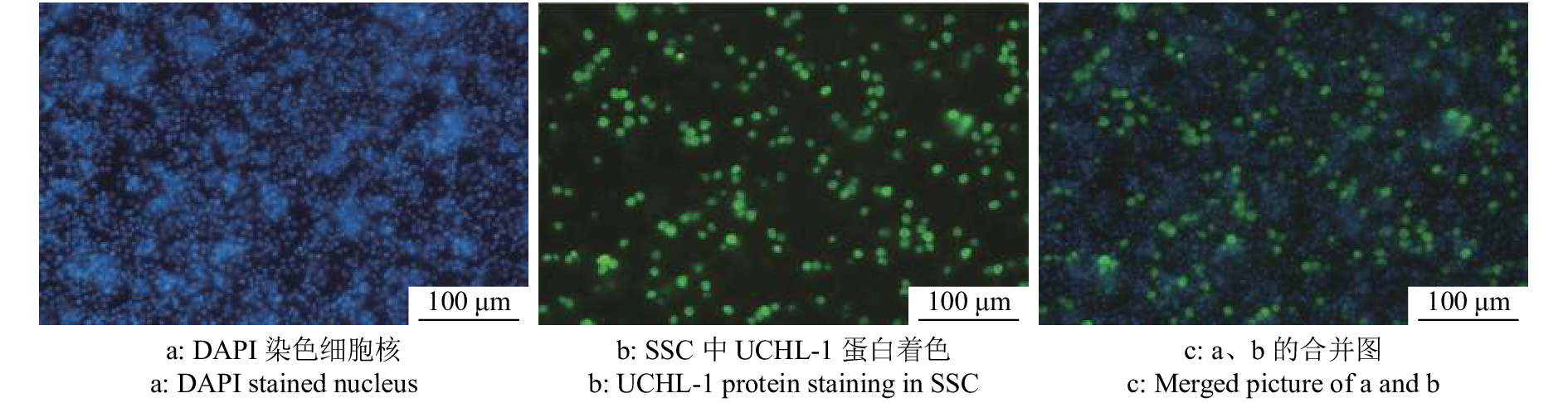
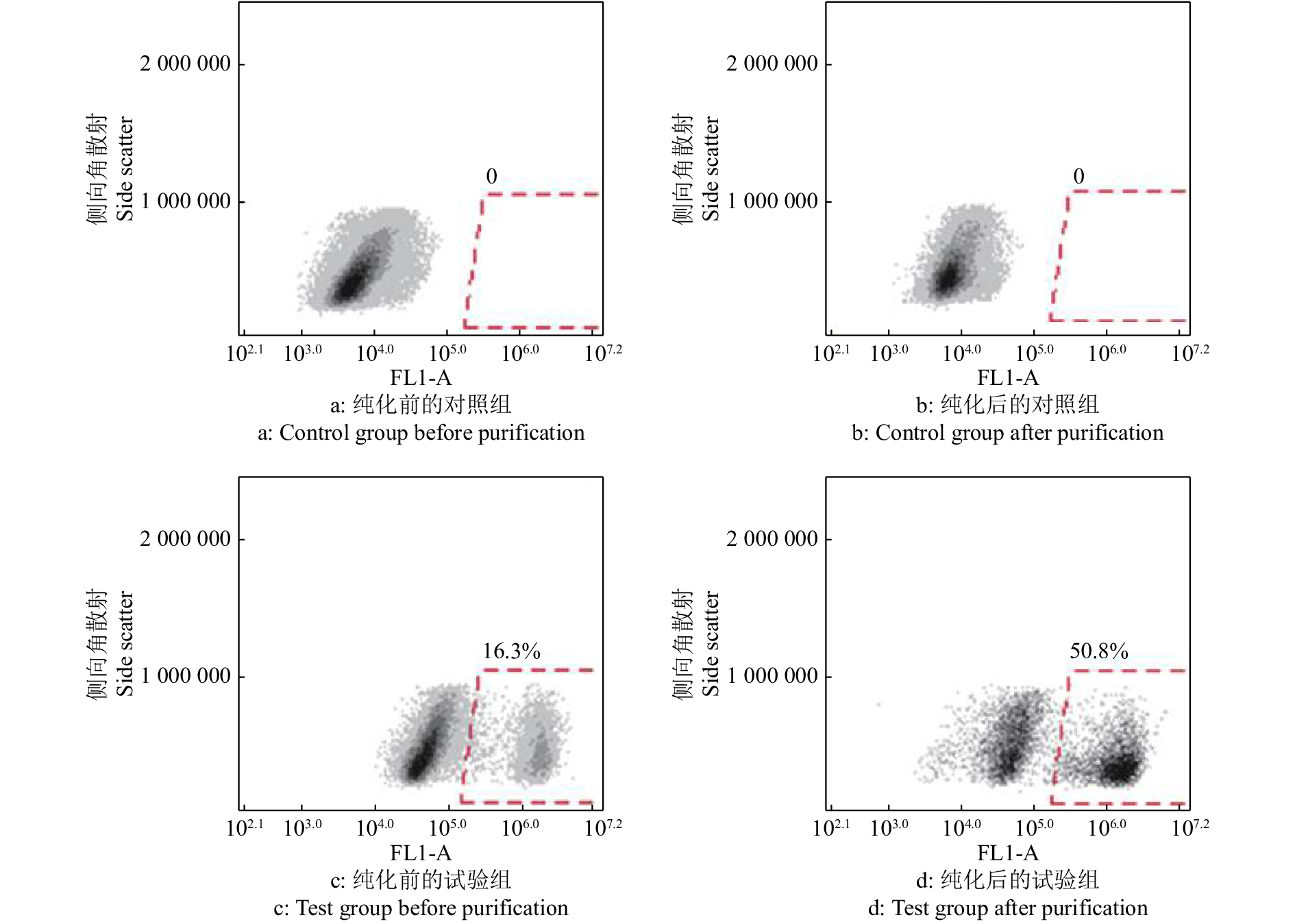
大白仔猪SSC的分离仍采用应用较为广泛的两步酶消化法,经两步酶消化分离得到睾丸单细胞悬液(图3a),SSC呈圆形或椭圆形,比其他体细胞大。经过明胶铺板过夜处理后,收集未贴壁的细胞重新铺板(图3b),此时的细胞悬液纯度相较于差速贴壁前的单细胞悬液已大幅提高。同时,取纯化后单细胞悬液进行免疫荧光染色以及流式细胞术分析。免疫荧光染色结果表明,纯化得到的大部分细胞呈UCHL-1抗体染色阳性(图4),显示成功分离纯化获得SSC。而且,流式细胞术分析的结论也佐证了免疫荧光染色的结果,初始分离得到的单细胞悬液中SSC占比为16.3%,经过纯化后提高到了50.8%(图5),这些都表明我们制备的供体SSC悬液可以用于之后的移植试验。
![]() 图 5 流式细胞术分析纯化前后睾丸单细胞悬液中精原干细胞(SSC)的比例对照组未经UCHL-1抗体染色,试验组经由UCHL-1抗体染色;各图中,横坐标FL1-A表示绿色荧光通道(Alexa Flour 488);0、0、16.3%和50.8%表示各处理细胞群中绿色荧光标记的阳性细胞占比Figure 5. Flow cytometry analysis of the proportion of spermatogonial stem cells (SSCs) in testicular single cell suspension before and after purificationThe control group was not stained with UCHL-1 antibody, and the test group was stained; In each graph, the horizontal axis FL1-A indicates the green fluorescence channel (Alexa Flour 488); 0, 0, 16.3% and 50.8% indicate the proportion of positive cells with green fluorescent labels in cell population
图 5 流式细胞术分析纯化前后睾丸单细胞悬液中精原干细胞(SSC)的比例对照组未经UCHL-1抗体染色,试验组经由UCHL-1抗体染色;各图中,横坐标FL1-A表示绿色荧光通道(Alexa Flour 488);0、0、16.3%和50.8%表示各处理细胞群中绿色荧光标记的阳性细胞占比Figure 5. Flow cytometry analysis of the proportion of spermatogonial stem cells (SSCs) in testicular single cell suspension before and after purificationThe control group was not stained with UCHL-1 antibody, and the test group was stained; In each graph, the horizontal axis FL1-A indicates the green fluorescence channel (Alexa Flour 488); 0, 0, 16.3% and 50.8% indicate the proportion of positive cells with green fluorescent labels in cell population2.3 猪SSC的移植及鉴定
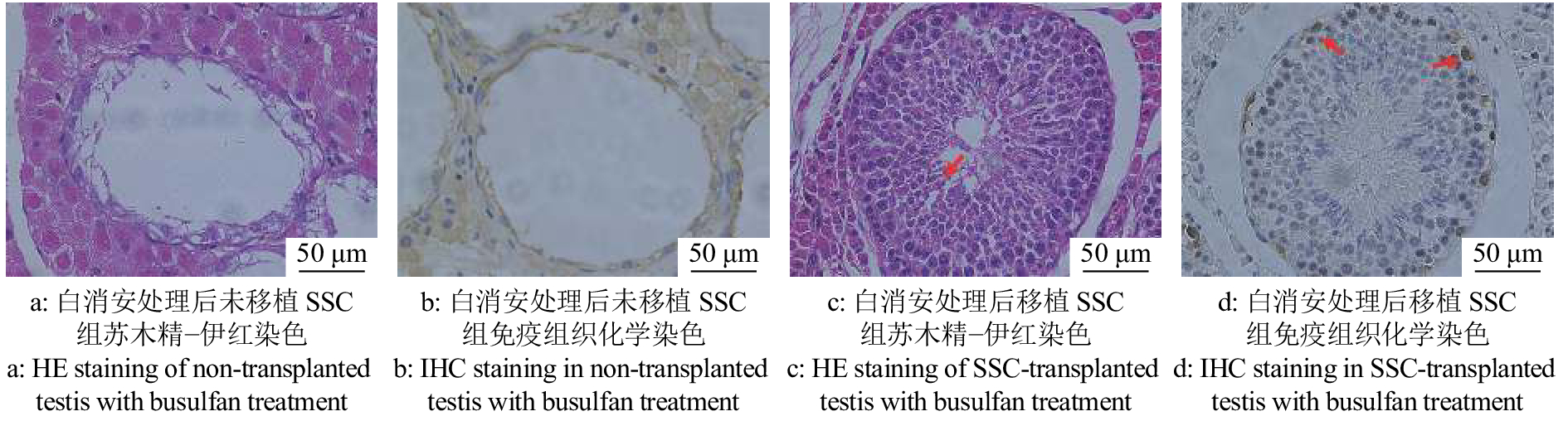
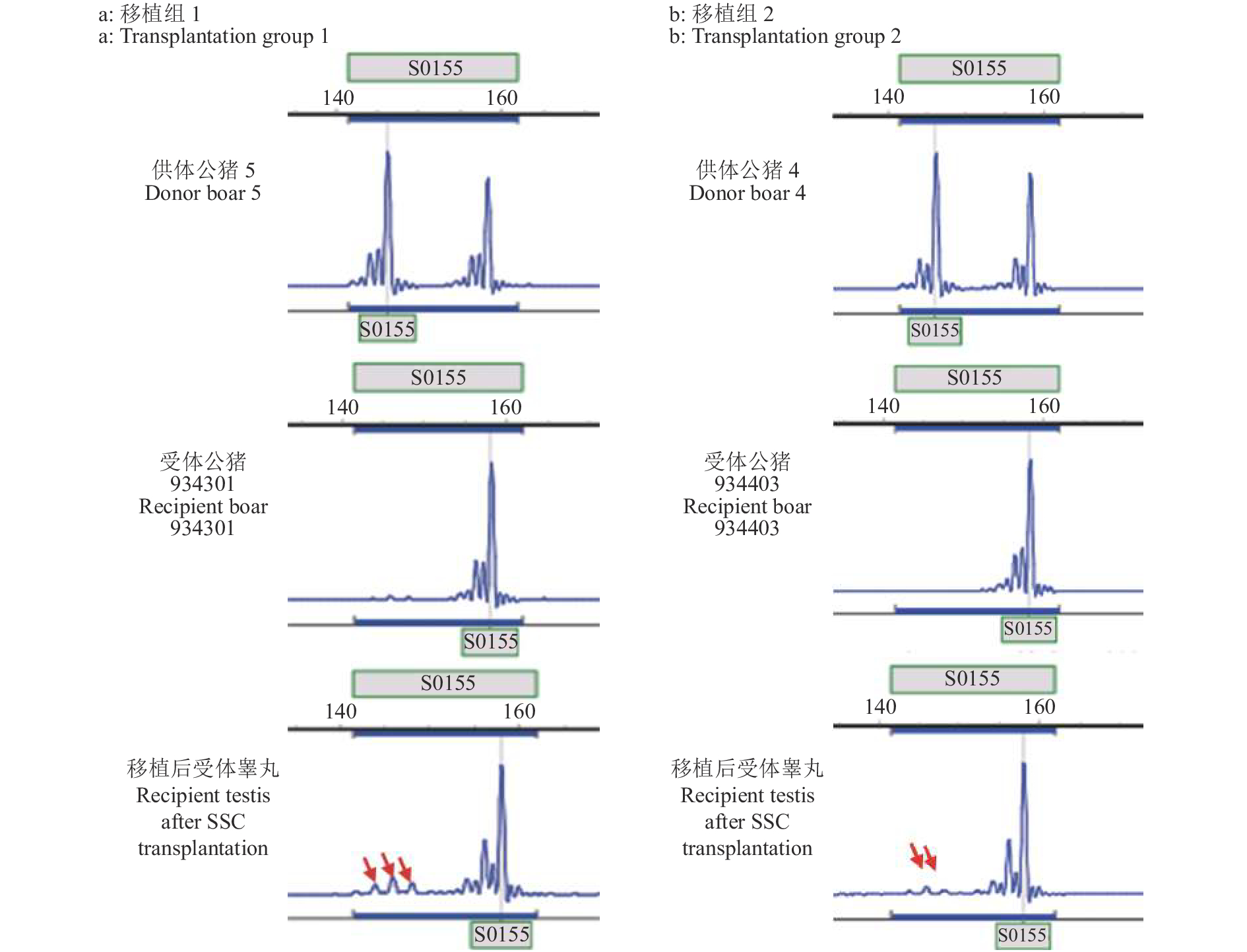
纯化后的猪SSC悬液在37 ℃条件下带往猪场进行移植试验。经SSCT 4个月后,采集受体睾丸组织进行HE和IHC染色分析。与注射白消安但未进行SSC移植组相比,SSC移植组HE染色结果显示受体公猪睾丸内部分曲细精管结构完整且有各级生精细胞的存在,同时观察到了未成熟的精子细胞(图6a、6c)。同时IHC染色结果表明受体睾丸曲细精管基底膜上具有UCHL-1阳性的精原细胞(图6b、6d)。为了探究曲细精管基底膜上的SSC是来源于供体还是受体公猪自身恢复的内源性的SSC,对3头受体公猪睾丸组织DNA、供体基因组DNA以及受体组织DNA进行微卫星标记分析。结果显示,耳号为934301公猪的睾丸组织DNA的微卫星标记测序峰图在S0155位点与供体组织基因组DNA(编号为供体5)有部分吻合,耳号为934403公猪的睾丸组织DNA的微卫星标记测序峰图在S0155位点供体组织基因组DNA(编号为供体4)有部分吻合(图7)。这些结果表明,受体公猪睾丸内的确有供体SSC的定植,供体来源的SSC可以在受体睾丸中定植并存活超过4个月。同时我们对试验组6头受体公猪进行精液采集。待其达到性成熟时,经数次调教有3头可以采集到精液,其中有1头公猪的精液无精子存在。对2头公猪的精液DNA进行微卫星标记分析,结果并未在精液DNA中观测到供体来源的基因型信号。这一结果可能是由于移植到受体睾丸中的供体SSC并未大量分化为成熟精子,同时受体睾丸在SSCT后一直到性成熟采精的这一段时间内,白消安的药物作用逐渐减小造成了受体公猪睾丸内源性SSC的逐渐恢复也影响了外源SSC的有效增殖和分化。
![]() 图 6 移植后受体睾丸精原干细胞(SSC)的恢复c中红色箭头指向未成熟精子细胞,d中红色箭头指向位于曲细精管基底膜上的SSCFigure 6. Recovery of spermatogonial stem cells (SSCs) in testis of recipients after transplantationRed arrow in figure c points at immature sperm cells, and red arrow in figure d points at SSCs located on the basement membrane of seminiferous tubules
图 6 移植后受体睾丸精原干细胞(SSC)的恢复c中红色箭头指向未成熟精子细胞,d中红色箭头指向位于曲细精管基底膜上的SSCFigure 6. Recovery of spermatogonial stem cells (SSCs) in testis of recipients after transplantationRed arrow in figure c points at immature sperm cells, and red arrow in figure d points at SSCs located on the basement membrane of seminiferous tubules![]() 图 7 精原干细胞(SSC)移植后受体睾丸组织微卫星标记分析图中红色箭头指示在S0155位点出现供体基因型信号;S0355、Sw857位点结果不能区分供体和受体基因型Figure 7. Microsatellite marker analysis of testes of recipients with spermatogonial stem cell (SSC) transplantationThe red arrows in the figure indicate that the donor genotype signal appears at S0155;The microsatellite results of S0355 and Sw857 loci cannot distinguish the genotype of donor and recipient
图 7 精原干细胞(SSC)移植后受体睾丸组织微卫星标记分析图中红色箭头指示在S0155位点出现供体基因型信号;S0355、Sw857位点结果不能区分供体和受体基因型Figure 7. Microsatellite marker analysis of testes of recipients with spermatogonial stem cell (SSC) transplantationThe red arrows in the figure indicate that the donor genotype signal appears at S0155;The microsatellite results of S0355 and Sw857 loci cannot distinguish the genotype of donor and recipient3. 讨论与结论
猪作为重要的农业资源以及生物医学研究的理想动物模型,一方面为人类提供大部分的肉食,另一方面由于其与人类具有高度相似的解剖学结构和生理学特点而被广泛应用于生物医学等领域的研究[40]。人工授精(Artificial insemination,AI)是生猪生产实践中主要的繁殖手段,而SSCT的进一步开发与应用将提供一种新型高效的种猪繁殖替代策略,可显著提高种猪的繁殖与生产效率[34]。另外,当前制备转基因猪主要采用的是体细胞核移植技术(Somatic cell nuclear transfer,SCNT),但这一技术需要熟练的显微注射和胚胎操作且克隆胚胎发育效率较低,而SSCT的应用可以规避与胚胎操作和克隆胚胎重编程异常相关的问题,即在猪SSC上引入遗传修饰并进行移植以连续产生含有遗传修饰的精子用于配种进而获得转基因猪,所以SSCT在生产转基因猪方面也有着巨大的潜力。尽管SSCT具有着巨大的应用价值,但其在大动物上的研究却进展缓慢,至今也只有山羊[12]、绵羊[17]和树鼩[27]产生了供体SSC来源的后代,而在猪SSCT上的研究也报道较少[5-8]。前人的研究表明,能否制备理想的内源性SSC消融的移植受体是阻滞SSCT进一步发展的关键因素,而白消安处理受体是相对可行的方法,包括直接注射低剂量白消安到妊娠后期母猪子宫内导致内源性生殖细胞的减少且没有出现明显的健康问题[8, 38],或是直接将较高剂量的白消安直接注射到受体公猪睾丸内导致内源性精子发生的耗竭以及睾丸体细胞的应答反应为SSCT提供定植所需的微环境“niche”[37]。本研究采用易于在猪场实施的白消安注射法,直接将白消安注射到公猪的睾丸中,并且在注射后对受体睾丸组织进行HE染色以及IHC处理,结果表明,获得了具有内源性SSC消融的“唯支持细胞”表型的受体猪。虽然试验公猪血常规检测表明,在白消安注射后的一段时间内会影响公猪的造血功能,但这种影响会随着猪只日龄的增长而逐渐减弱,同时试验组公猪与对照组公猪的表型并无差异且没有出现明显的健康问题,因此可以用来制备SSCT受体猪。
其次,猪SSC分离纯化过程中得到高密度、高纯度、高活力的单细胞悬液也是成功移植的前提条件。当前应用于猪SSC分离的方法较多,主要有差速贴壁法[41-42]、Staput速度沉降[7]、不连续的Percoll密度梯度离心法[8]、基于SSC表面标记的荧光激活细胞分选法(Fluorescence-activated cell sorting,FACS)[43]和免疫磁珠激活细胞分选法(Magnetic-activated cell sorting,MACS)[44]。但是由于猪SSC缺乏独特的分子标记以及猪源抗体,限制了FACS和MACS的广泛应用。而密度梯度离心因离心过程繁复容易引起SSC的丢失。与前人的研究相比,本研究采用了较为简单且最大程度保存SSC高活力的差速贴壁法。经过多次差速贴壁,将除SSC以外的其他体细胞大多保留在明胶包被的细胞培养板上,进而获得较高纯度、较高活力的SSC进行后续的移植试验。
为了检测SSCT后受体睾丸中能否重新建立起供体细胞来源的精子发生,一般采用诸如PKH26等荧光染料示踪、微卫星标记分析或使用转基因和报告基因等方法。Honaramooz等[5]将红色荧光染料PKH26与供体细胞共孵育来标记供体细胞,在超声引导下通过睾丸网注射供体细胞悬液,研究发现供体细胞在约50%的曲细精管中存活了超过4周,但由于其采用的供体生殖细胞悬液是未经纯化的各种细胞混合物以及受体是未经任何处理的青春期前公猪,因此并未观察到供体来源的精子发生。虽然理论上如果将受体睾丸中的内源性生殖细胞耗竭可以在一定程度上提高供体来源干细胞的定植效率,但之前在淋巴干细胞上的研究表明,供体来源的干细胞能与受体动物中的内源性干细胞有效竞争生存所需的微环境“niche”[45],这提示我们SSCT的关键或许是分离纯化到较高活性的供体SSC。Mikkola等[6]将未纯化的供体睾丸生殖细胞(含SSC)移植到2头遗传性短尾精子缺陷(Immotile short-tail sperm,ISTS)的公猪睾丸内,移植后在1头公猪睾丸内观察到了供体来源的精子发生以及运动的精子,移植27周后,基因分型检测结果也证实了供体基因型来源的精子的产生。2013年,Zeng等[7]使用AAV载体将报告基因EGFP体外转染到猪生殖细胞中,并将表达EGFP的生殖细胞移植到经白消安处理的受体睾丸中,观察到了转染的阳性生殖细胞在受体睾丸内定植以及重新启动供体来源的精子发生的过程,并获得了供体来源的转基因精子,进行体外受精得到了表达EGFP的转基因胚胎。Kim等[8]使用慢病毒载体将报告基因EGFP转染到供体SSC中,随后将EGFP表达阳性的SSC移植到内源性SSC消融的受体猪睾丸内,成功获得了转基因精子,且通过卵胞浆内单精子注射(Intra cytoplasmic sperm injection,ICSI)技术使卵子受精。参照前人的研究成果,本研究采用微卫星标记分析来鉴定采集到的精液以及受体睾丸组织中是否存在供体来源的基因型,在相应的微卫星位点观察供、受体基因组DNA和受体睾丸组织或精液DNA的差异。微卫星标记分析的结果表明,供体来源的SSC在受体睾丸中成功定植了4个月,这与前人的研究结论相一致。然而,我们的精液微卫星标记分析结果显示,在移植后受体精液中并未检测到供体来源的精子。这或许是由于移植到受体睾丸中的供体SSC并未大量分化为成熟精子,同时白消安的药物作用随时间逐渐减小造成了受体公猪睾丸内源性SSC的逐渐恢复也影响了外源SSC的有效增殖和进一步的分化。
本研究的结果证实了睾丸组织注射白消安可以有效去除猪内源SSC,可以用来制备SSCT受体猪。SSCT后,供体来源的SSC可以在内源性SSC消融的受体睾丸中定植存活超过4个月。下一步工作需要进一步探讨优化供体SSC的制备和移植方式、白消安处理的流程,并对移植中可能存在的免疫排斥反应进行研究,以期提高猪SSCT的效率。
致谢:感谢温氏集团种猪科技公司在试验中给予的支持和帮助!
-
图 5 流式细胞术分析纯化前后睾丸单细胞悬液中精原干细胞(SSC)的比例
对照组未经UCHL-1抗体染色,试验组经由UCHL-1抗体染色;各图中,横坐标FL1-A表示绿色荧光通道(Alexa Flour 488);0、0、16.3%和50.8%表示各处理细胞群中绿色荧光标记的阳性细胞占比
Figure 5. Flow cytometry analysis of the proportion of spermatogonial stem cells (SSCs) in testicular single cell suspension before and after purification
The control group was not stained with UCHL-1 antibody, and the test group was stained; In each graph, the horizontal axis FL1-A indicates the green fluorescence channel (Alexa Flour 488); 0, 0, 16.3% and 50.8% indicate the proportion of positive cells with green fluorescent labels in cell population
图 6 移植后受体睾丸精原干细胞(SSC)的恢复
c中红色箭头指向未成熟精子细胞,d中红色箭头指向位于曲细精管基底膜上的SSC
Figure 6. Recovery of spermatogonial stem cells (SSCs) in testis of recipients after transplantation
Red arrow in figure c points at immature sperm cells, and red arrow in figure d points at SSCs located on the basement membrane of seminiferous tubules
图 7 精原干细胞(SSC)移植后受体睾丸组织微卫星标记分析
图中红色箭头指示在S0155位点出现供体基因型信号;S0355、Sw857位点结果不能区分供体和受体基因型
Figure 7. Microsatellite marker analysis of testes of recipients with spermatogonial stem cell (SSC) transplantation
The red arrows in the figure indicate that the donor genotype signal appears at S0155;The microsatellite results of S0355 and Sw857 loci cannot distinguish the genotype of donor and recipient
表 1 微卫星多态性分析引物序列
Table 1 Primer sequences for polymorphic microsatellites analysis
位点名称
Locus name所在染色体
Chr.引物序列1)(5′→3′)
Primer sequence退火温度/ ℃
Annealing temperature等位基因长度范围/bp
Allele rangeS0155 1 D-TGTTCTCTGTTTCTCCTCTGTTTG
AAAGTGGAAAGAGTCAATGGCTAT55 142~162 S0355 15 D-TCTGGCTCCTACACTCCTTCTTGATG
TTGGGTGGGTGCTGAAAAATAGGA55 244~271 Sw857 14 D-TGAGAGGTCAGTTACAGAAGACC
GATCCTCCTCCAAATCCCAT55 141~159 1)字母“D”表示带荧光标记的引物
1) Letter“D” indicates dye labelled primer -
[1] KERR J, LOVELAND K, OBRYAN M, et al. Cytology of the testis and intrinsic control mechanisms[M]//NEILL J D. Knobil and Neill’s Physiology of Reproduction. Amsterdam: Elsevier, 2006: 827-947.
[2] MULDER C L, ZHENG Y, JAN S Z, et al. Spermatogonial stem cell autotransplantation and germline genomic editing: A future cure for spermatogenic failure and prevention of transmission of genomic diseases[J]. Human Reproduction Update, 2016, 22(5): 561-573. doi: 10.1093/humupd/dmw017
[3] BRINSTER R L, ZIMMERMANN J W. Spermatogenesis following male germ-cell transplantation[J]. Proceedings of the National Academy of Sciences of the United States of America, 1994, 91(24): 11298-11302. doi: 10.1073/pnas.91.24.11298
[4] BRINSTER R L, AVARBOCK M R. Germline transmission of donor haplotype following spermatogonial transplantation[J]. Proceedings of the National Academy of Sciences of the United States of America, 1994, 91(24): 11303-11307. doi: 10.1073/pnas.91.24.11303
[5] HONARAMOOZ A, MEGEE S O, DOBRINSKI I. Germ cell transplantation in pigs[J]. Biology of Reproduction, 2002, 66(1): 21-28. doi: 10.1095/biolreprod66.1.21
[6] MIKKOLA M, SIRONEN A, KOPP C, et al. Transplantation of normal boar testicular cells resulted in complete focal spermatogenesis in a boar affected by the immotile short-tail sperm defect[J]. Reproduction in Domestic Animals, 2006, 41(2): 124-128. doi: 10.1111/j.1439-0531.2006.00651.x
[7] ZENG W X, TANG L, BONDAREVA A, et al. Viral transduction of male germline stem cells results in transgene transmission after germ cell transplantation in pigs[J]. Biology of Reproduction, 2013, 88(1): 27.
[8] KIM B G, KIM Y H, LEE Y A, et al. Production of transgenic spermatozoa by lentiviral transduction and transplantation of porcine spermatogonial stem cells[J]. Tissue Engineering and Regenerative Medicine, 2014, 11(6): 458-466. doi: 10.1007/s13770-014-0078-8
[9] IZADYAR F, DEN OUDEN K, STOUT T A E, et al. Autologous and homologous transplantation of bovine spermatogonial stem cells[J]. Reproduction, 2003, 126(6): 765-774. doi: 10.1530/rep.0.1260765
[10] HERRID M, VIGNARAJAN S, DAVEY R, et al. Successful transplantation of bovine testicular cells to heterologous recipients[J]. Reproduction, 2006, 132(4): 617-624. doi: 10.1530/rep.1.01125
[11] STOCKWELL S, HERRID M, DAVEY R, et al. Microsatellite detection of donor-derived sperm DNA following germ cell transplantation in cattle[J]. Reproduction, Fertility and Development, 2009, 21(3): 462-468. doi: 10.1071/RD08130
[12] HONARAMOOZ A, BEHBOODI E, MEGEE S O, et al. Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats[J]. Biology of Reproduction, 2003, 69(4): 1260-1264. doi: 10.1095/biolreprod.103.018788
[13] HONARAMOOZ A, BEHBOODI E, BLASH S, et al. Germ cell transplantation in goats[J]. Molecular Reproduction and Development, 2003, 64(4): 422-428. doi: 10.1002/mrd.10205
[14] KAUL G, KAUR J, RAFEEQI T A. Ultrasound guided transplantation of enriched and cryopreserved spermatogonial cell suspension in goats[J]. Reproduction in Domestic Animals, 2010, 45(6): e249-e254. doi: 10.1111/j.1439-0531.2009.01549.x
[15] RODRIGUEZ-SOSA J R, DOBSON H, HAHNEL A. Isolation and transplantation of spermatogonia in sheep[J]. Theriogenology, 2006, 66(9): 2091-2103. doi: 10.1016/j.theriogenology.2006.03.039
[16] HERRID M, DAVEY R, STOCKWELL S, et al. A shorter interval between irradiation of recipient testis and germ cell transplantation is detrimental to recovery of fertility in rams[J]. International Journal of Andrology, 2011, 34(5 Pt 1): 501-512.
[17] HERRID M, OLEJNIK J, JACKSON M, et al. Irradiation enhances the efficiency of testicular germ cell transplantation in sheep[J]. Biology of Reproduction, 2009, 81(5): 898-905. doi: 10.1095/biolreprod.109.078279
[18] STOCKWELL S, HILL J R, DAVEY R, et al. Transplanted germ cells persist long-term in irradiated ram testes[J]. Animal Reproduction Science, 2013, 142(3/4): 137-140. doi: 10.1016/j.anireprosci.2013.09.012
[19] SCHLATT S, FOPPIANI L, ROLF C, et al. Germ cell transplantation into X-irradiated monkey testes[J]. Human Reproduction, 2002, 17(1): 55-62. doi: 10.1093/humrep/17.1.55
[20] SCHLATT S, ROSIEPEN G, WEINBAUER G F, et al. Germ cell transfer into rat, bovine, monkey and human testes[J]. Human Reproduction, 1999, 14(1): 144-150. doi: 10.1093/humrep/14.1.144
[21] JAHNUKAINEN K, EHMCKE J, QUADER M A, et al. Testicular recovery after irradiation differs in prepubertal and pubertal non-human primates, and can be enhanced by autologous germ cell transplantation[J]. Human Reproduction, 2011, 26(8): 1945-1954. doi: 10.1093/humrep/der160
[22] HERMANN B P, SUKHWANI M, WINKLER F, et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm[J]. Cell Stem Cell, 2012, 11(5): 715-726. doi: 10.1016/j.stem.2012.07.017
[23] GOOSSENS E, TOURNAYE H. Functional sperm produced after spermatogonial stem cell transplantation into rhesus[J]. Asian Journal of Andrology, 2013, 15(2): 216-217. doi: 10.1038/aja.2012.155
[24] SHETTY G, MITCHELL J M, MEYER J M, et al. Restoration of functional sperm production in irradiated pubertal rhesus monkeys by spermatogonial stem cell transplantation[J]. Andrology, 2020, 8(5): 1428-1441. doi: 10.1111/andr.12807
[25] KIM Y, TURNER D, NELSON J, et al. Production of donor-derived sperm after spermatogonial stem cell transplantation in the dog[J]. Reproduction, 2008, 136(6): 823-831. doi: 10.1530/REP-08-0226
[26] HARKEY M A, ASANO A, ZOULAS M E, et al. Isolation, genetic manipulation, and transplantation of canine spermatogonial stem cells: progress toward transgenesis through the male germ-line[J]. Reproduction, 2013, 146(1): 75-90. doi: 10.1530/REP-13-0086
[27] LI C H, YAN L Z, BAN W Z, et al. Long-term propagation of tree shrew spermatogonial stem cells in culture and successful generation of transgenic offspring[J]. Cell Research, 2017, 27(2): 241-252. doi: 10.1038/cr.2016.156
[28] HERRID M, NAGY P, JUHASZ J, et al. Donor sperm production in heterologous recipients by testis germ cell transplantation in the dromedary camel[J]. Reproduction, Fertility and Development, 2019, 31(3): 538-546. doi: 10.1071/RD18191
[29] GUL M, HILDORF S, DONG L, et al. Review of injection techniques for spermatogonial stem cell transplantation[J]. Human Reproduction Update, 2020, 26(3): 368-391. doi: 10.1093/humupd/dmaa003
[30] GONZÁLEZ R, DOBRINSKI I. Beyond the mouse monopoly: Studying the male germ line in domestic animal models[J]. ILAR Journal, 2015, 56(1): 83-98. doi: 10.1093/ilar/ilv004
[31] KUBOTA H, BRINSTER R L. Spermatogonial stem cells[J]. Biology of Reproduction, 2018, 99(1): 52-74. doi: 10.1093/biolre/ioy077
[32] SAVVULIDI F, PTACEK M, SAVVULIDI VARGOVA K, et al. Manipulation of spermatogonial stem cells in livestock species[J]. Journal of Animal Science and Biotechnology, 2019, 10: 46. doi: 10.1186/s40104-019-0355-4
[33] OGAWA T, ARÉCHAGA J M, AVARBOCK M R, et al. Transplantation of testis germinal cells into mouse seminiferous tubules[J]. International Journal of Developmental Biology, 1997, 41(1): 111-122.
[34] GIASSETTI M I, CICCARELLI M, OATLEY J M. Spermatogonial stem cell transplantation: Insights and outlook for domestic animals[J]. Annual Review of Animal Biosciences, 2019, 7: 385-401. doi: 10.1146/annurev-animal-020518-115239
[35] OATLEY J M. Recent advances for spermatogonial stem cell transplantation in livestock[J]. Reproduction, Fertility and Development, 2017, 30(1): 44-49.
[36] IWAMOTO T, HIRAKU Y, OIKAWA S, et al. DNA intrastrand cross-link at the 5'-GA-3' sequence formed by busulfan and its role in the cytotoxic effect[J]. Cancer Science, 2004, 95(5): 454-458. doi: 10.1111/j.1349-7006.2004.tb03231.x
[37] LIN Z H, BAO J J, KONG Q F, et al. Effective production of recipient male pigs for spermatogonial stem cell transplantation by intratesticular injection with busulfan[J]. Theriogenology, 2017, 89: 365-373. doi: 10.1016/j.theriogenology.2016.10.021
[38] HONARAMOOZ A, BEHBOODI E, HAUSLER C L, et al. Depletion of endogenous germ cells in male pigs and goats in preparation for germ cell transplantation[J]. Journal of Andrology, 2005, 26(6): 698-705. doi: 10.2164/jandrol.05032
[39] FAO/ISAG. Secondary guidelines for development of national farm animal genetic resources management plans measurement of domestic animal diversity (MoDAD): Recommended microsatellite markers[EB/OL]. Joint ISAG/FAO Standing Committee. 2004, (2004-09-09)[2020-05-01]. http://wwwuser.gwdg.de/~uatz/FAO/cattel.htm (5 of 5)09/09/2004 16: 53: 05.
[40] YANG H Q, WU Z F. Genome editing of pigs for agriculture and biomedicine[J]. Frontiers in Genetics, 2018, 9: 360. doi: 10.3389/fgene.2018.00360
[41] WANG X Y, CHEN T F, ZHANG Y N, et al. Isolation and culture of pig spermatogonial stem cells and their in vitro differentiation into neuron-like cells and adipocytes[J]. International Journal of Molecular Sciences, 2015, 16(11): 26333-26346. doi: 10.3390/ijms161125958
[42] ZHENG Y, TIAN X E, ZHANG Y Q, et al. In vitro propagation of male germline stem cells from piglets[J]. Journal of Assisted Reproduction and Genetics, 2013, 30(7): 945-952. doi: 10.1007/s10815-013-0031-0
[43] KIM Y H, KIM B J, KIM B G, et al. Stage-specific embryonic antigen-1 expression by undifferentiated spermatogonia in the prepubertal boar testis[J]. Journal of Animal Science, 2013, 91(7): 3143-3154. doi: 10.2527/jas.2012-6139
[44] ZHANG P F, QIN Y W, ZHENG Y, et al. Phospholipase D family member 6 is a surface marker for enrichment of undifferentiated spermatogonia in prepubertal boars[J]. Stem Cells and Development, 2018, 27(1): 55-64. doi: 10.1089/scd.2017.0140
[45] QUESENBERRY P J, STEWART F M, ZHONG S J, et al. Lymphohematopoietic stem cell engraftment[J]. Annals of the New York Academy of Sciences, 1999, 872: 40-47. doi: 10.1111/j.1749-6632.1999.tb08451.x
-
期刊类型引用(0)
其他类型引用(1)



 下载:
下载: