Biological effect and targeted mutation screening of space-induced mutagenesis and heavy ion radiation in rice
-
摘要:目的
从不同诱变世代比较空间诱变和重离子诱变对水稻生物学效应及后代变异频率的差异,为水稻诱变育种提供一定的方法和理论指导。
方法对水稻纯系品种‘华航31号’干种子进行空间诱变和不同剂量重离子诱变处理,以未诱变处理的种子为对照,对诱变一代进行表型及细胞学诱变效应的分析;对诱变二代直链淀粉含量及粒型性状进行表型和基因型定向筛选,比较2种诱变处理的性状变异频率。
结果空间诱变一代的种子活力指数比对照下降了14.62%;重离子诱变一代的种子活力指数随辐射剂量增加呈现马鞍型效应曲线,其中10 Gy重离子辐射剂量下种子活力指数比对照下降了14.92%,与空间诱变的诱变效应相近。空间诱变二代粒型和直链淀粉含量的突变频率分别为4.14%和1.61%,80 Gy重离子辐射诱变二代粒型和直链淀粉含量的突变频率分别为4.88%和1.55%。利用HRM技术扫描了水稻直链淀粉含量Wx基因的4个位点共673 bp序列,在空间诱变的4 736份样本中发现3个SNP变异,突变密度为1/1063.83 kb;在重离子诱变4 848份样本中发现4个SNP变异,突变密度为1/815.68 kb。
结论2种诱变处理均可诱发水稻产生性状变异。空间诱变的生理损伤类似于低剂量重离子辐射,而诱发变异的频率与高剂量重离子辐射相近。
Abstract:ObjectiveTo compare the biological effects and variation frequency of two mutagenesis methods, including space mutation and heavy ion radiation, in different generations of rice (Oryza sativa L.), and provide some methods and theoretical guidances for rice mutation breeding.
MethodThe dry seeds of pure line rice variety ‘Huahang 31’ were treated using space mutation and different dose of heavy ion radiation, and the seeds without mutation treatment were used as the control. The phenotypic and cytological mutagenic effects of the 1st generation of mutation (M1) were analyzed. The amylose content and grain shape trait of the 2nd generation of mutation (M2) were screened by phenotypic and genotypic targeted screening. The variation frequencies in M2 of two mutation treatments were compared.
ResultThe seed vigor index of space mutation M1 was 14.62% lower than that of the control. The seed vigor index of heavy ion radiation M1 showed a saddle effect curve with the increase of radiation dose, and the seed vigor index of 10 Gy heavy ion radiation M1 was 14.92% lower than that of the control which was like the mutation effect of space mutation. The grain type and amylose content mutation frequencies of space-induced M2 were 4.14% and 1.61% respectively, while the grain type and amylose content mutation frequencies of 80 Gy heavy ion radiation were 4.88% and 1.55% respectively. When HRM technology was used to scan the 673 bp sequences of four intervals of Wx gene, three SNP mutations were found in 4 736 samples of space-induced with a mutation density of 1/1063.83 kb. Four SNP mutations were found in 4 848 samples of heavy ion radiation with a mutation density of 1/815.68 kb.
ConclusionTwo kinds of mutagenic treatments can induce the character variations of rice. The physiological effects of space-induced mutagenesis M1 is similar to low-dose heavy ion radiation, and the mutation frequency of M2 is similar to high-dose heavy ion radiation.
-
Keywords:
- rice /
- space mutation /
- heavy ion radiation /
- biological effect /
- mutant screening
-
-
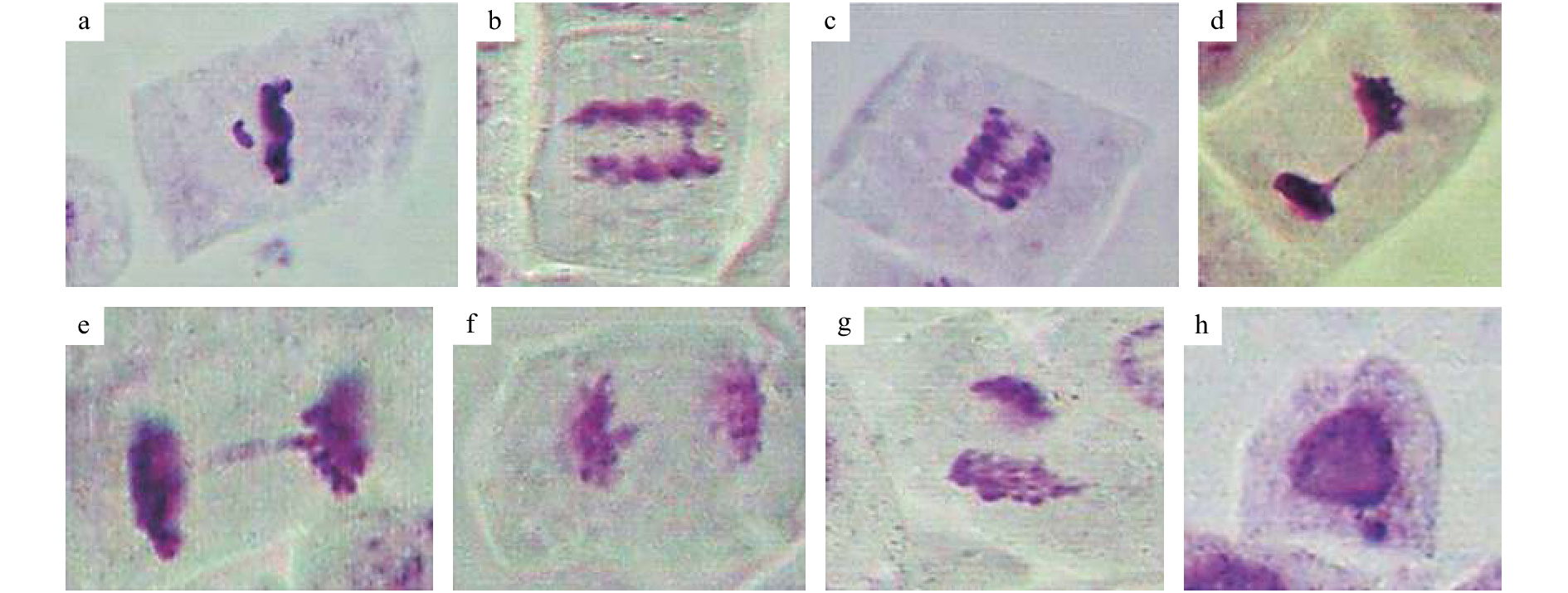
图 2 诱变处理后水稻M1代植株根尖细胞有丝分裂染色体异常
a:断片;b:单桥;c:多桥;d,e:后期单桥;f:染色体落后;g:不均等分裂;h:微核
Figure 2. Chromosomal abnormality of apical mitosis of root tip cells of contemporary rice plants after mutagenic treatment
a: Fragment; b: Single bridge; c: Polybridge; d,e: Single bridge at anaphase; f: Chromosome lag; g: Unequal cell division; h: Micronucleus
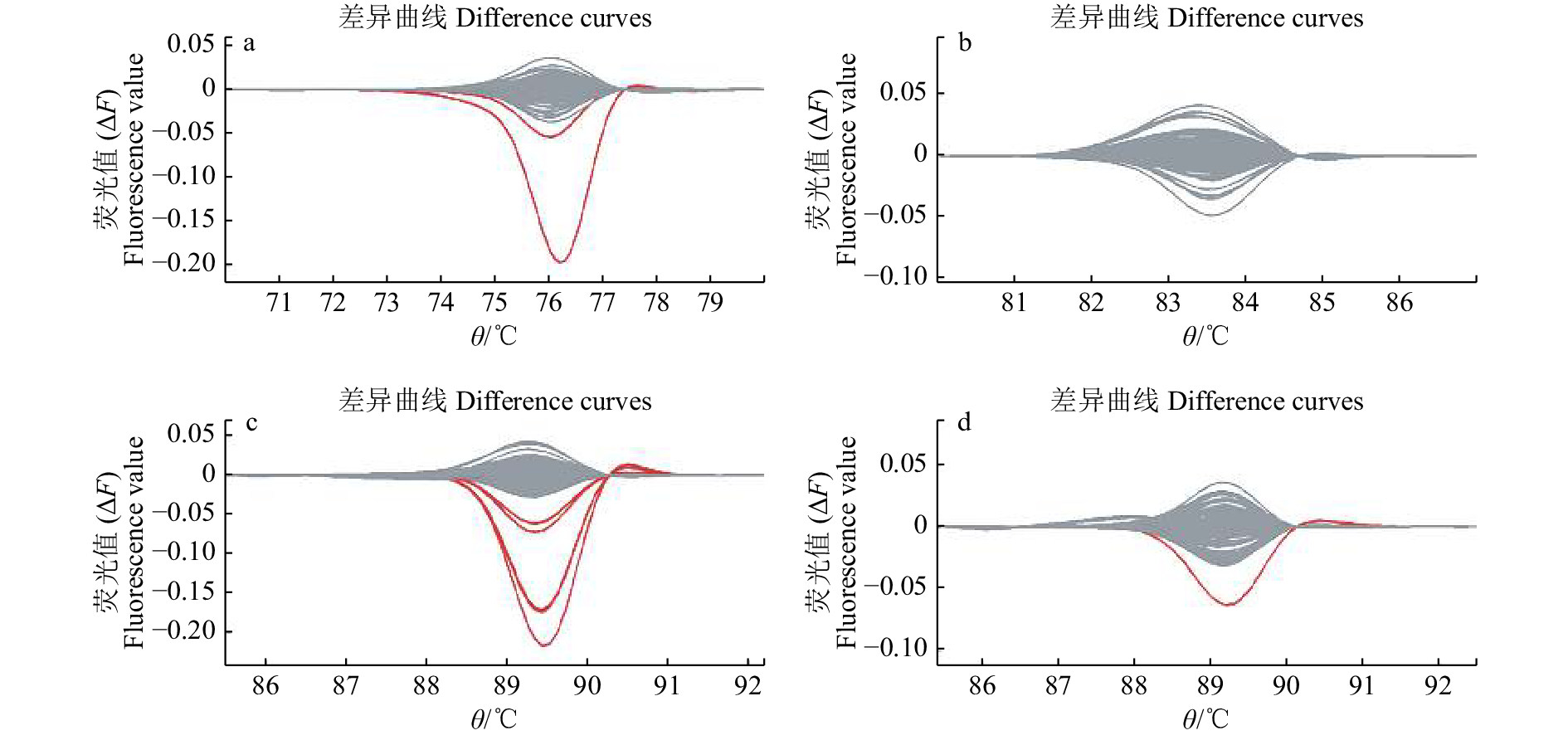
图 4 DNA混合池的4对巢式PCR引物扩增产物的HRM检测结果
a:Wxab-O和Wxab-I引物扩增产物检测;b:EX4-O和EX4-I引物扩增产物检测;c:EX9-O和EX9-I引物扩增产物检测;d:EX10-O和EX10-I引物扩增产物检测
Figure 4. HRM detections of four pairs of nested PCR primer amplification products in DNA mixing cell
a: Detecting amplifications of primers Wxab-O and Wxab-I; b: Detecting amplifications of primers EX4-O and EX4-I; c: Detecting amplifications of primers EX9-O and EX9-I; d: Detecting amplifications of primers EX10-O and EX10-I
图 5 重离子诱变exon 10突变体序列分析结果
a:检测区域的序列分析,外显子区域由绿色标注,差异位点由红色标注;b:exon 10翻译的蛋白序列分析;c:对照Sanger测序峰图;d:突变体Sanger测序峰图
Figure 5. The results of sequence analysis of exon 10 mutant induced by heavy ion
a: Sequence analysis, green for exon and red for mutation site; b: Protein sequence of exon 10; c: Sanger sequencing results of CK; d: Sanger sequencing results of the mutant
表 1 用于HRM检测的特异引物信息
Table 1 Specific primer information for HRM detection
引物名称
Primer
name检测位点
Detection
site前引物(5′→3′)
Former
primer后引物(5′→3′)
Latter
primer片段大小/bp
Fragment
sizeWxab-O Wxa与Wxb
差异位点GAGGGAGAGGGGGAGAGAGAGATC TCCAGCCCAACACCTTACAGAAAT 348 Wxab-I CTTCACTTCTCTGCTTGTGTTGTT CATGTGATCGATCTGAATAAGAGG 110 EX4-O exon 4 CAGATCATCACAAGATCGATTAGC AACCTGAAATCACCAGTGGAAG 286 EX4-I CCAAGTTTTTGTGGTGCAATTC TAAGCTCAGTCCAACTGCTAAATG 204 EX9-O exon 9 CGACGGGTATGAGTAAGATTCTAAG CATTCGTTCTTACCGTGGTTG 384 EX9-I AGATCCTTTTGAGCTGACAACC GTACTTGGCGGTGATGTACTTG 294 EX10-O exon 10 AGAACGAATGCATTCTTCACAAGA GCCTCACCCCTTCTAATTATTTGA 421 EX10-I ACAAGGCAAGAATGAGTGACAA GCATATCGTGCAAGTGTGTCTT 247 表 2 空间诱变及重离子诱变对种子活力的影响1)
Table 2 Effects of space mutation and heavy ion mutation on seed vigor
处理
Treatment发芽势/%
Germination potential发芽率/%
Germination rate芽长/cm
Bud length发芽指数
Germination index活力指数
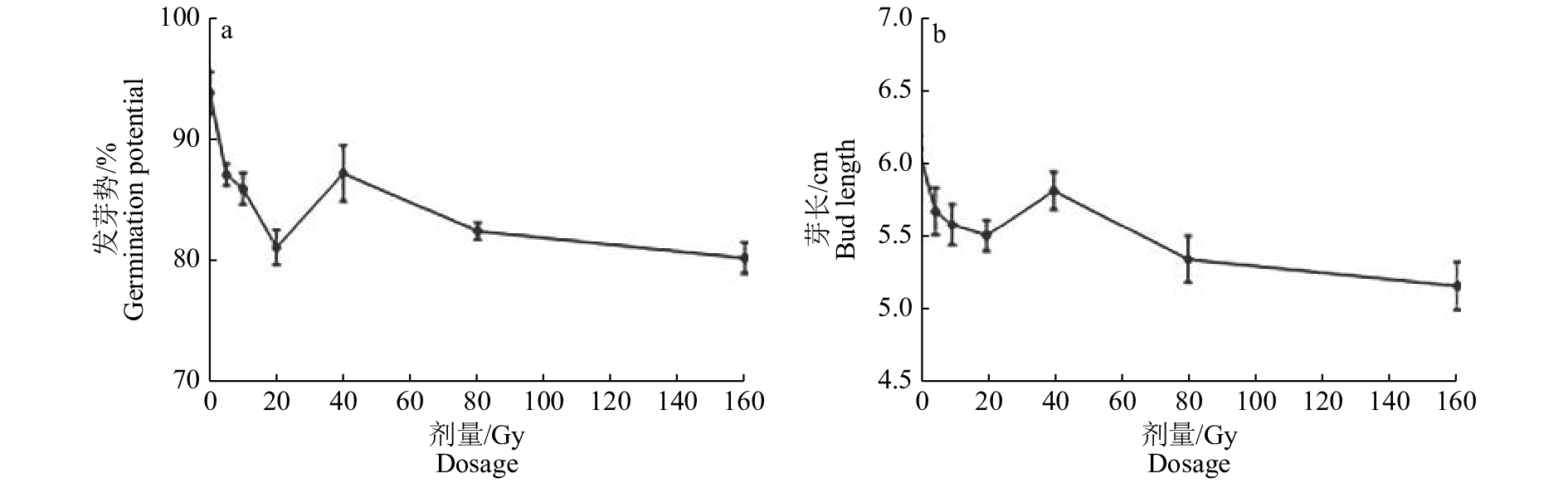
Vigor indexCK 94.02±2.45 (0.00) 100.00±0.00 (0.00) 6.11±0.09 (0.00) 37.19 (0.00) 227.32 (0.00) SP 100.00±0.00 (6.36) 100.00±0.00 (0.00) 5.10±0.08** (−16.53) 38.06 (2.34) 194.08 (−14.62) 5 87.29±1.29 (−7.17) 97.07±0.07 (−2.93) 5.69±0.16* (−6.98) 36.38 (−2.18) 206.85 (−9.01) 10 86.14±1.82* (−8.39) 95.15±3.00 (−4.85) 5.59±0.14* (−8.48) 34.58 (−7.04) 193.40 (−14.92) 20 81.32±2.01** (−13.51) 96.20±1.95 (−3.80) 5.52±0.11** (−9.72) 33.80 (−9.12) 186.52 (−17.95) 40 87.43±3.27 (−7.02) 95.37±2.30 (−4.63) 5.83±0.13 (−4.67) 35.70 (−4.01) 208.00 (−8.50) 80 82.69±0.97** (−12.06) 96.29±2.75 (−3.71) 5.35±0.16** (−12.42) 34.39 (−7.55) 184.09 (−19.02) 160 80.47±1.83** (−14.42) 95.56±2.36 (−4.44) 5.17±0.16** (−15.39) 32.87 (−11.62) 169.99 (−25.22) 1) CK: 对照;SP: 空间诱变;5、10、20、40、80和160分别代表重离子辐射不同剂量(Gy);括号内数据为相对效应,相对效应=(处理−对照)/对照×100%;“*”“**”分别表示与CK在0.05、0.01水平差异显著
1) CK: Control; SP: Space-induced mutation; 5,10,20,40,80 and 160 represented different doses of heavy ion radiation(Gy) respectively; The data in brackets were relative effects,relative effect=(treatment – CK)/CK ×100%;“*”and“**”represented significant differences with the control at the levels of 0.05 and 0.01 respectively表 3 空间诱变和重离子诱变的水稻种子M1代植株主要农艺性状 1)
Table 3 The main agronomic characters of contemporary plants of rice seeds treated by space mutation and heavy ion mutation
处理
Treatment株高/cm
Plant
height穗长/cm
Panicle
length有效穗数
No. of productive
panicle每穗粒数
No. of spikelet
per panicle结实率/%
Setting
percentage谷粒长宽比
Grain length-
width ratio千粒质量/g
1000-grain
weightCK 119.17±1.48 30.22±0.81 8.33±1.45 232.51±11.95 80.03±1.18 3.99±0.02 21.47±0.13 SP 120.30±1.90 26.50±0.54** 8.40±0.51 184.92±7.62** 84.94±1.61 3.98±0.06 21.18±0.42 5 121.20±1.46 27.33±0.61** 10.00±0.45 161.29±14.02** 84.56±0.54 4.00±0.02 21.88±0.16 10 121.60±1.21 28.50±0.54 7.60±0.68 193.85±14.00** 84.55±2.61 3.96±0.01 22.00±0.17 20 121.40±0.98 28.47±0.33 8.00±0.71 193.24±6.63** 84.02±1.75 3.92±0.02 22.24±0.07 40 118.40±0.81 28.34±0.61 8.40±0.81 183.72±9.25** 82.63±1.61 3.92±0.05 22.00±0.25 80 121.25±0.63 28.76±0.32 8.25±0.48 201.59±14.05** 63.13±5.36** 3.94±0.03 21.83±0.17 160 121.30±1.02 26.79±0.87** 7.20±0.66 189.71±14.31** 48.69±5.13** 3.93±0.02 21.36±0.34 1) CK: 对照;SP: 空间诱变;5、10、20、40、80和160分别代表重离子辐射不同剂量(Gy);表中数据为平均值±标准误,“**”表示与CK在0.01水平差异显著
1)CK: Control; SP: Space-induced mutation; 5,10,20,40,80 and 160 represented different doses of heavy ion radiation (Gy) respectively;The data in the table were mean value ± standard error,and“**”represented significant difference with the control at the level of 0.01表 4 空间诱变和重离子诱变对水稻M1代植株根尖细胞的影响1)
Table 4 Effects of space mutation and heavy ion mutation on root tip cells of contemporary rice plants
处理
Treatment有丝分裂指数/%
Mitotic
index微核千分率/‰
Permillage of
micronucleus染色体畸变率/%
Aberration rate
of chromosome染色体畸变类型2)Aberrant types of chromosome 双桥或多桥
Double bridge
or poly bridge断片
Fragment落后染色体
Laggard
chromosome不均等分裂
Unequal cell
divisionCK 5.32±0.27 0.38±0.18 1.25±0.50 1(0.25) 4(1.00) 0(0) 0(0) SP 5.26±0.31 1.12±0.32 3.28±0.92* 1(0.25) 9(2.00) 4(0.75) 1(0.25) 5 5.10±0.34 1.23±0.47 5.25±0.92* 1(0.25) 11(2.75) 3(0.75) 1(0.25) 10 5.15±0.34 1.82±0.44 6.25±1.08** 5(1.25) 8(2.00) 8(2.00) 2(0.50) 20 4.90±0.32 2.56±0.46* 7.00±1.17** 4(1.00) 16(4.00) 5(1.25) 0(0) 40 4.63±0.24 3.61±0.72** 7.75±1.43** 4(1.00) 19(4.75) 4(1.00) 2(0.50) 80 4.29±0.56 4.43±0.59** 10.00±0.58** 6(1.50) 20(5.00) 5(1.25) 6(1.50) 160 3.89±0.26* 5.81±0.84** 13.25±1.82** 6(1.50) 16(4.00) 10(2.50) 16(4.00) 1) CK: 对照;SP: 空间诱变;5、10、20、40、80和160分别代表重离子辐射不同剂量(Gy);表中数据为平均值±标准误,“*”“**”分别表示与CK在0.05、0.01水平差异显著;2) 表中括号里的数值表示各畸变类型的细胞数(括号外数值)占所观察分裂期细胞总数的百分比(%)
1) CK: Control; SP: Space-induced mutation; 5, 10, 20, 40, 80 and 160 represented different doses of heavy ion radiation (Gy) respectively; The data in the table was mean value ± standard error, “*” and “**” represented significant differences with the control at the levels of 0.05 and 0.01 respectively; 2) The values in brackets represented the ratios of the number of abnormal cells (values outside brackets) to total cells in cell division(%)表 5 空间诱变及重离子诱变水稻M2代粒型变异
Table 5 Grain type variation in the second generation of rice seeds treated by space mutation and heavy ion mutation
处理1)
Treatment粒型
Grain type平均值( $\bar x $)
Average
value标准差(s)
Standard
deviation变异幅度
Range of
variation变异系数/%
Coefficient of
variationF 筛选范围2)
Range of screening< $\bar x -3s$ > $\bar x+3s $ SP 粒长 Grain length/mm 9.69 0.134 9 8.70~11.00 1.39 0.67 粒宽 Grain width/mm 2.35 0.023 0 2.20~2.65 0.98 2.95 粒长宽比 Grain length to width 4.13 0.064 7 3.70~4.68 1.57 0.08 M 粒长 Grain length/mm 9.72 0.144 1 9.05~12.00 1.48 0.52 粒宽 Grain width/mm 2.35 0.035 2 2.00~3.00 1.50 0.51 粒长宽比 Grain length to width 4.13 0.087 7 3.08~5.11 2.12 0.05 CK 粒长 Grain length/mm 9.69 0.102 9 9.50~9.85 1.06 <9.3764 >9.9936 粒宽 Grain width/mm 2.35 0.033 3 2.30~2.40 1.42 <2.2500 >2.4500 粒长宽比 Grain length to width 4.12 0.073 4 4.02~4.28 1.78 <3.9017 >4.3424 1)SP: 空间诱变,M:80 Gy重离子诱变,CK: 对照;2) $\bar x $:对照的平均值
1) SP:Space-induced mutation, M: 80 Gy heavy ion mutation, CK: Control; 2) $\bar x $: Mean value of the control表 6 空间诱变和重离子诱变水稻M2代直链淀粉含量变异
Table 6 Variation of amylose content in the second generation of rice seeds treated by space mutation and heavy ion mutation
处理1)
Treatmentw(直链淀粉)/%
Amylose content标准差(s)
Standard deviation变异幅度
Range of variation变异系数/%
Coefficient of variationF 筛选范围2)
Range of screening< $\bar x-3s $ > $\bar x+3s $ SP 20.78 2.8957 11.91~30.81 13.94 2.97 M 20.80 2.8458 11.64~30.74 13.68 2.51 CK 20.92 2.2281 16.87~25.92 10.65 <14.239 7 >27.608 2 1) SP: 空间诱变,M:80 Gy重离子诱变,CK: 对照;2) $\bar x $:对照的平均值
1) SP:Space-induced mutation, M: 80 Gy heavy ion mutation, CK: Control; 2) $\bar x $: Mean value of the control -
[1] ZHAO K, TUNG C W, EIZENGA G C, et al. Genome-wide association mapping reveals a rich genetic architecture of complex traits in Oryza sativa[J]. Nature Communications, 2011, 2(1): 467. doi: 10.1038/ncomms1467
[2] GLASZMANN J C, KILIAN B, UPADHYAYVA H D, et al. Accessing genetic diversity for crop improvement[J]. Current Opinion in Plant Biology, 2010, 13(2): 167-173. doi: 10.1016/j.pbi.2010.01.004
[3] 刘录祥, 王晶, 赵林姝, 等. 作物空间诱变效应及其地面模拟研究进展[J]. 核农学报, 2004, 18(4): 247-251. doi: 10.3969/j.issn.1000-8551.2004.04.002 [4] 周利斌, 李文建, 曲颖, 等. 重离子束辐照育种研究进展及发展趋势[J]. 原子核物理评论, 2008(2): 71-76. [5] 陈志强, 郭涛, 刘永柱, 等. 水稻航天育种研究进展与展望[J]. 华南农业大学学报, 2009, 30(1): 7-11. [6] 李东芳, 倪丕冲, 沈桂芳. 水稻航天诱变育种及其机理研究的进展与展望[J]. 生物技术通报, 2004(3): 23-25. doi: 10.3969/j.issn.1002-5464.2004.03.006 [7] 樊秋玲, 刘敏. 空间育种研究进展[J]. 航天医学与医学工程, 2002, 15(3): 231-234. doi: 10.3969/j.issn.1002-0837.2002.03.020 [8] 史金铭. 空间和重离子辐射环境的诱变效应与DNA甲基化变化的关联[D]. 哈尔滨: 哈尔滨工业大学, 2010. [9] OGATA T, TESHIMA T, KAGAWA K, et al. Particle irradiation suppresses metastatic potential of cancer cells[J]. Cancer Research, 2005, 65(1): 113-120.
[10] SHI J M, LU W H, SUN Y Q. Comparison of space flight and heavy ion radiation induced genomic/epigenomic mutations in rice (Oryza sativa)[J]. Life Sciences in Space Research, 2014(1): 74-79.
[11] WEI L, YANG Q, XIA H, et al. Analysis of cytogenetic damage in rice seeds induced by energetic heavy ions on-ground and after spaceflight[J]. Journal of Radiation Research, 2006, 47(3/4): 273-278. doi: 10.1269/jrr.0613
[12] 王巍. 空间诱导水稻蛋白变化特征及空间辐射地基模拟诱因分析[D]. 哈尔滨: 哈尔滨工业大学, 2011. [13] 严文潮, 徐建龙, 俞法明, 等. 不同早籼基因型水稻的空间诱变效应研究[J]. 核农学报, 2004, 18(3): 174-178. doi: 10.3969/j.issn.1000-8551.2004.03.005 [14] 刘向东, 卢永根. 整体荧光原位杂交用于检测水稻特异DNA程序的研究[J]. 华南农业大学学报, 1998, 19(3): 1-4. [15] BUSH S M, KRYSAN P J. ITILLING: A personalized approach to the identification of induced mutations in Arabidopsis[J]. Plant Physiology, 2010, 154(1): 25-35. doi: 10.1104/pp.110.159897
[16] SIMKO I. High-resolution DNA melting analysis in plant research[J]. Trends in Plant Science, 2016, 21(6): 528-537. doi: 10.1016/j.tplants.2016.01.004
[17] 马良勇, 季芝娟, 曾宇翔, 等. 不同航天器搭载对籼稻诱变效果的比较[J]. 核农学报, 2011, 25(1): 7-11. [18] 刘永柱, 许立超, 郭涛, 等. 2个三系杂交稻保持系航天诱变效应的研究[J]. 华南农业大学学报, 2013, 34(3): 292-299. doi: 10.7671/j.issn.1001-411X.2013.03.003 [19] 俞法明, 严文潮, 毛雪琴, 等. 利用空间诱变技术进行早籼稻新品种的改良[J]. 核农学报, 2014, 28(6): 949-954. doi: 10.11869/j.issn.100-8551.2014.06.0949 [20] 黄永相, 郭涛, 蔡金洋. 空间环境和60Co-γ辐照对水稻稻米品质的诱变效应[J]. 核农学报, 2013, 27(6): 709-714. doi: 10.11869/hnxb.2013.06.0709 [21] XU X, LIU B, ZHANG L, et al. Mutagenic effects of heavy ion irradiation on rice seeds[J]. Nuclear Instruments & Methods in Physics Research, 2012, 290(1): 19-25.
[22] 卢明. 低能重离子C和Li及Co-γ射线对水稻的生物学效应研究[D]. 长沙: 湖南农业大学, 2005. [23] 梅曼彤, 邓红, 卢永根. 高能重离子辐射对水稻的生物学效应[J]. 作物学报, 1995, 21(3): 307-314. doi: 10.3321/j.issn:0496-3490.1995.03.008 [24] 程维民, 刘斌美, 叶亚峰. 重离子辐照创建水稻直链淀粉、蛋白质突变系的研究[J]. 中国农学通报, 2016, 6(32): 86-90. [25] 杨瑰丽, 陈莹, 郭涛, 等. 碳离子束辐照水稻诱变效应及突变体的筛选[J]. 华南农业大学学报, 2018, 39(2): 29-33. doi: 10.7671/j.issn.1001-411X.2018.02.005 [26] MANI S, TABIL L G, SOKHANSANJ S. Mechanical properties of corn stover grind[J]. Transactions of the ASAE, 2004, 47(6): 1983-1990. doi: 10.13031/2013.17786
[27] 易继财, 庄楚雄, 姚涓, 等. 空间搭载诱导水稻种子突变的分子标记多态性分析[J]. 生物物理学报, 2002, 18(4): 478-483. doi: 10.3321/j.issn:1000-6737.2002.04.019 [28] 骆艺, 王旭杰, 梅曼彤, 等. 空间搭载水稻种子后代基因组多态性及其与空间重离子辐射关系的探讨[J]. 生物物理学报, 2006, 22(2): 131-138. doi: 10.3321/j.issn:1000-6737.2006.02.010 [29] 刘自增, 吴周良, 阎萍, 等. 高分辨率熔解曲线分析应用的研究进展[J]. 中国畜牧兽医, 2013, 40(8): 105-111. doi: 10.3969/j.issn.1671-7236.2013.08.024 [30] 李贵成, 王林辉, 罗红兵. 重离子辐射玉米种子的细胞学观察[J]. 湖南农业大学学报(自然科学版), 2007, 33(5): 48-50. [31] LANDAU A, LENCINA F, PACHECO M G, et al. Plastome mutations and recombination events in barley chloroplast mutator seedlings[J]. Journal of Heredity, 2016, 107(3): 266-273. doi: 10.1093/jhered/esw003
[32] BOTTICELLA E, SESTILI F, HEMANDEZ-LOPEZ A, et al. High resolution melting analysis for the detection of EMS induced mutations in wheat SbeIIa genes[J]. BMC Plant Biology, 2011, 11(1): 1-14. doi: 10.1186/1471-2229-11-1
[33] KIM S I, TAI T H. Identification of novel rice low phytic acid mutations via TILLING by sequencing[J]. Molecular Breeding, 2014, 34(4): 1731-1732. doi: 10.1007/s11032-014-0187-z
[34] 王彩芬, 马晓玲, 安永平, 等. TILLING术在水稻耐盐基因SKC1突变体筛选中的应用[J]. 作物杂志, 2013(5): 66-70. doi: 10.3969/j.issn.1001-7283.2013.05.019 [35] HWANG J E, JANG D S, LEE K J, et al. Identification of gamma ray irradiation-induced mutations in membrane transport genes in a rice population by TILLING[J]. Genes & Genetic Systems, 2016, 91(5): 245-256.
[36] DU Y, LI W, YU L, et al. Mutagenic effects of carbon-ion irradiation on dry Arabidopsis thaliana seeds[J]. Mutation Research, 2014, 759: 28-36. doi: 10.1016/j.mrgentox.2013.07.018



 下载:
下载: