Effect of orally administrated chlortetracycline microcapsule on microflora quantity in sheep rumen
-
摘要:目的
评估口服自主研制的金霉素微囊化颗粒对绵羊瘤胃微生物菌群数量的影响。
方法选取体质量(29.0±2.3) kg、6月龄左右、体况良好的18只绵羊,随机分为6组,包括1个空白对照组和5个给药组,每组3只。空白对照组只饲喂基础饲粮;5个给药组分别为试验I组:基础饲粮+普通金霉素预混剂颗粒,试验II组:基础饲粮+氢化油处方组金霉素微囊化颗粒,试验III组:基础饲粮+PEG4000/单甘脂(质量比为5∶5)处方组金霉素微囊化颗粒,试验IV组:基础饲粮+氢化油/单甘脂(质量比为9∶1)处方组金霉素微囊化颗粒,试验V组:基础饲粮+氢化油/单甘脂(质量比为8∶2)处方组金霉素微囊化颗粒。给药组绵羊金霉素最终给药剂量均为25 mg/kg,每天给药1次且均在晨饲前灌胃给药,连续给药5 d,通过瘤胃液采样器采集瘤胃内容物。采集的瘤胃内容物经4层纱布过滤后,收集滤液装于冻存管内,所有样品经液氮速冻后置于−80 ℃保存,运用实时荧光定量PCR(RT-PCR)技术对绵羊瘤胃液相瘤胃微生物进行定量检测。
结果与普通金霉素预混剂颗粒相比,氢化油/单甘脂(质量比为9∶1)处方组金霉素微囊化颗粒有较好效果,只显著增加了产琥珀酸拟杆菌的数量,对绵羊瘤胃其他微生物菌群数量影响均不显著;与空白对照组相比,氢化油/单甘脂(质量比为9∶1)处方组金霉素微囊化颗粒及PEG4000/单甘脂(质量比为5∶5)处方组金霉素微囊化颗粒有相对较好效果,分别只对牛链球菌及产琥珀酸拟杆菌的数量有显著降低趋势,对绵羊瘤胃其他微生物菌群数量影响均不显著。
结论氢化油/单甘脂(质量比为9︰1)处方组金霉素微囊化颗粒对绵羊瘤胃微生物数量抑制作用较小,符合临床应用要求。
Abstract:ObjectiveTo evaluate the effect of orally administrated self-developed chlortetracycline microcapsule on microflora quantity in sheep rumen.
MethodEighteen sheep of (29.0±2.3) kg of 6-month-old with good health condition were randomly assigned to six groups with three replicates in eash grcup, including one control group and five medicated groups. The sheep in the control group were only fed with basal diet. The treatments in five medicated groups were as follows. Group I: Basal diet supplemented with normal chlortetracycline premix granule; Group II: Basal diet supplemented with hydrogenated oil prescription chlortetracycline microcapsule; Group III: Basal diet supplemented with PEG4000/glycerol stearate (mass ratio of 5︰5) prescription chlortetracycline microcapsule; Group IV: Basal diet supplemented with hydrogenated oil/glycerol stearate (mass ratio of 9︰1) prescription chlortetracycline microcapsule; Group V: Basal diet supplemented with hydrogenated oil/glycerol stearate (mass ratio of 8︰2) prescription chlortetracycline microcapsule. The chlortetracycline administration dosages of sheep in the five medicated groups were all 25 mg/kg. The sheep were administrated once a day before morning feeding for five days. The rumen contents were collected by rumen fluid collector and quickly filtered through four layers of gauze after continuous administration. The filtrate were collected in the cryopreserved tube and quickly frozened by liquid nitrogen then stored at −80 ℃. The rumen microorganisms of liquid phase were quantitatively detected by real-time fluorescence quantitative PCR (RT-PCR).
ResultCompared with the normal chlortetracycline premix granule,hydrogenated oil/glycerol stearate (mass ratio of 9︰1) prescription chlortetracycline microcapsule had better effect, only significantly increased the quantity of Fibrobacter succinogens, and had no significant influence on the quantities of other microbial flora in sheep rumen. Compared with the control group, hydrogenated oil/glycerol stearate (mass ratio of 9︰1) prescription chlortetracycline microcapsule and PEG4000/glycerol stearate (mass ratio of 5︰5) prescription chlortetracycline microcapsule had relatively better effects, significantly decreased the quantities of Streptococcus bovis and F. succinogens respectively, and had no significant influence on the quantities of other microbial flora in sheep rumen.
ConclusionThe hydrogenated oil/glycerol stearate (mass ratio of 9︰1) prescription chlortetracycline microcapsule has less inhibition effect on rumen microflora quantity and meets clinical application requirements.
-
Keywords:
- sheep /
- rumen /
- microbe /
- chlortetracycline premix /
- chlortetracycline microcapsule
-
-
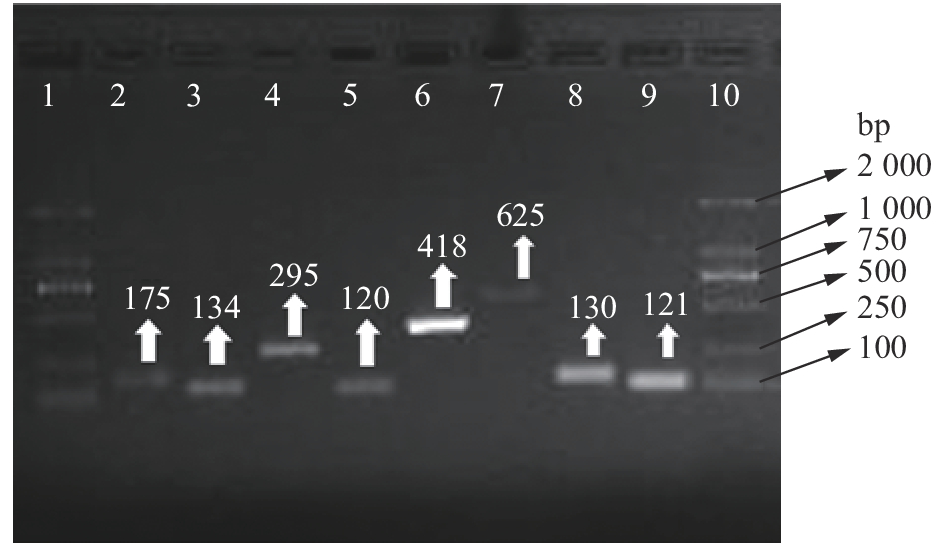
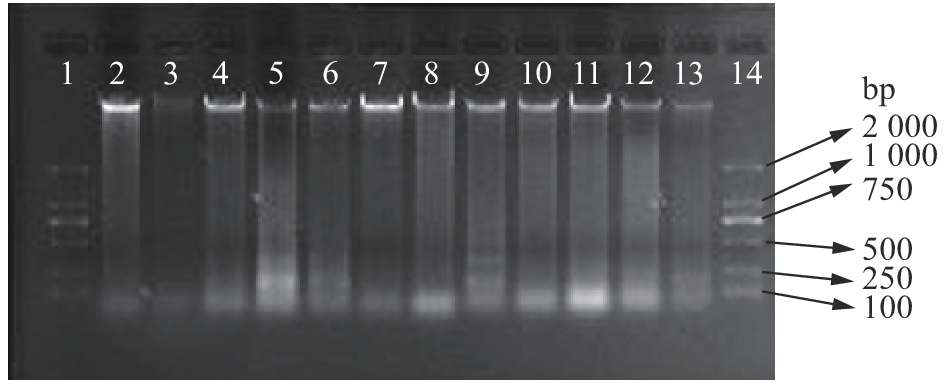
图 2 8种瘤胃微生物的普通PCR扩增结果
1、10:DL2000 marker,2:白色瘤胃球菌,3:牛链球菌,4:黄化瘤胃球菌,5:瘤胃厌氧真菌,6:普雷沃氏杆菌,7:溶纤维丁酸弧菌,8:瘤胃总细菌,9:产琥珀酸拟杆菌
Figure 2. Normal PCR amplification results of eight kinds of ruminal microbes
1, 10: DL2000 marker; 2: Ruminococcus albus; 3: Streptococcus bovis; 4: Ruminococcus flavefaciens; 5: Rumen anaerobic fungi; 6: Prevotella spp.; 7: Butyrivibrio fibrisolvens; 8: Rumen general bacteria; 9: Fibrobacter succinogens
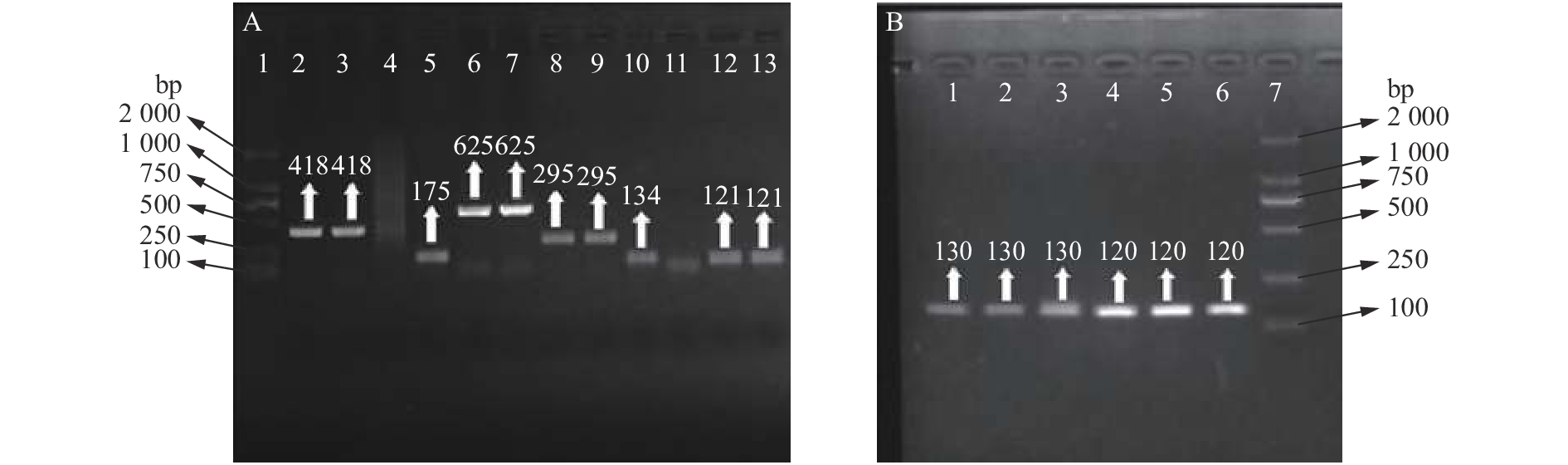
图 4 8种瘤胃微生物菌液PCR扩增
图A中,1:DL2000 marker,2、3:普雷沃氏杆菌,5:白色瘤胃球菌,6、7:溶纤维丁酸弧菌,8、9:黄化瘤胃球菌,10:牛链球菌,12、13:产琥珀酸拟杆菌;图B中,1、2、3:瘤胃总细菌,4、5、6:瘤胃厌氧真菌,7:DL2000 marker
Figure 4. Bacterial fluid PCR amplification of eight kinds of ruminal microbes
In figure A, 1: DL2000 marker; 2, 3: Prevotella spp.; 5: Ruminococcus albus; 6, 7: Butyrivibrio fibrisolvens; 8, 9: Ruminococcus flavefaciens; 10: Streptococcus bovis; 12, 13: Fibrobacter succinogens. In figure B, 1, 2, 3: Rumen general bacteria; 4, 5, 6: Rumen anaerobic fungi; 7: DL2000 marker
表 1 瘤胃微生物PCR扩增引物
Table 1 PCR amplification primers of ruminal microbes
引物名称
Primer name序列(5′→3′)
Primer sequenceθ退火/℃
Annealing temperature产物长度/bp
Product length参考文献
Reference瘤胃总细菌
Rumen general bacteriaF: CGGCAACGAGCGCAACCC
R: CCATTGTAGCACGTGTGTAGCC60 130 [7] 瘤胃厌氧真菌
Rumen anaerobic fungiF:GAGGAAGTAAAAGTCGTAACAAGGTTTC
R: CAAATTCACAAAGGGTAGGATGATT60 120 [7] 白色瘤胃球菌
Ruminococcus albusF: CCCTAAAAGCAGTCTTAGTTCG
R: CCTCCTTGCGGTTAGAACA55 175 [8] 黄化瘤胃球菌
Ruminococcus flavefaciensF: TCTGGAAACGGATGGTA
R: CCTTTAAGACAGGAGTTTACAA55 295 [8] 产琥珀酸拟杆菌
Fibrobacter succinogensF: GTTCGGAATTACTGGGCGTAAA
R: CGCCTGCCCCTGAACTATC60 121 [7] 牛链球菌
Streptococcus bovisF: ATTCTTAGAGATAGGGTTTCTCTT
R: ACCTTATGATGGCAACTAACAATA60 134 [9] 普雷沃氏菌属
Prevotella spp.F: GAA GGT CCC CCA CAT TG
R: CAA TCG GAG TTC TTC GTG56 418 [10] 溶纤维丁酸弧菌
Butyrivibrio fibrisolvensF: CGCATGATGCAGTGTGAAAAGCTC
R: CCTCCCGACACCTATTATTCATCG56 625 [9] 表 2 普通金霉素预混剂颗粒及4种金霉素微囊化颗粒对绵羊瘤胃微生物数量的影响
Table 2 Effects of normal chlortetracycline premix granule and four kinds of chlortetracycline microcapsules on the quantity of ruminal microbe of sheep
微生物种类
Microbe species微生物数量1) Microbe quantity I II III IV V CK 瘤胃总细菌 Rumen general bacteria 12.65Aa 12.24Aa 12.56Aa 12.43Aa 12.33Aa 12.35a 产琥珀酸拟杆菌 Fibrobacter succinogens 8.75BCcd 9.19Bbc 8.29Cd 10.14Aa 9.40Bb 10.21a 白色瘤胃球菌 Ruminococcus albus 8.78Aa 8.26Bb 9.03Aa 8.70Aa 8.77Aa 8.76a 黄化瘤胃球菌 Ruminococcus flavefaciens 9.79Aa 9.20Bb 9.82Aa 9.61Aa 9.61Aa 9.63a 普雷沃氏杆菌属 Prevotella spp. 11.53Aa 11.22Aa 11.40Aa 11.09Aa 11.46Aa 11.22a 溶纤维丁酸弧菌 Butyrivibrio fibrisolvens 9.00Bb 8.90Bb 10.50Aa 9.34Bb 8.92Bb 9.28b 牛链球菌 Streptococcus bovis 7.32Ab 7.41Ab 7.66Aab 7.21Ab 7.48Ab 8.04a 瘤胃厌氧真菌 Rumen anaerobic fungi 8.77Aa 8.35Aa 8.72Aa 8.98Aa 8.37Aa 8.82a 1) I组:基础饲粮+普通金霉素预混剂颗粒,II组:基础饲粮+氢化油处方组金霉素微囊化颗粒,III组:基础饲粮+PEG4000/单甘脂(质量比为5∶5)处方组金霉素微囊化颗粒,IV组:基础饲粮+氢化油/单甘脂(质量比为9∶1)处方组金霉素微囊化颗粒,V组:基础饲粮+氢化油/单甘脂(质量比为8∶2)处方组金霉素微囊化颗粒,CK:基础饲粮;相同微生物种类数据后的不同大写字母表示5个给药组间差异显著,不同小写字母表示5个给药组和空白对照组间差异显著(P<0.05,Duncan’s法)
1) Group I : Basal diet supplemented with normal chlortetracycline premix granule, group II : Basal diet supplemented with hydrogenated oil prescription chlortetracycline microcapsule, group III : Basal diet supplemented with PEG4000/glycerol stearate (mass ratio of 5∶5) prescription chlortetracycline microcapsule, group IV : Basal diet supplemented with hydrogenated oil/glycerol stearate (mass ratio of 9∶1) prescription chlortetracycline microcapsule, group V : Basal diet supplemented with hydrogenated oil/glycerol stearate (mass ratio of 8∶2) prescription chlortetracycline microcapsule, CK: Basal diet; Different capital letters after the same microbe species data indicate significant differences among five medicated groups, different lowercase letters after the same microbe species data indicate significant differences among five medicated groups and the blank control group (P<0.05, Duncan’s method) -
[1] ROSS E M, PETROVSKI S, MOATE P J, et al. Metagenomics of rumen bacteriophage from thirteen lactating dairy cattle[J]. BMC Microbiology, 2013, 13(1): 242. doi: 10.1186/1471-2180-13-242.
[2] KIM M, MORRISON M, YU Z. Status of the phylogenetic diversity census of ruminal microbiomes[J]. FEMS Microbiology Ecology, 2011, 76(1): 49-63. doi: 10.1111/j.1574-6941.2010.01029.x
[3] 李伟. β−内酰胺类抗生素有抗奶对犊牛瘤胃菌群结构及生长影响的研究[D]. 大庆: 黑龙江八一农垦大学, 2017. [4] 黄显会, 胡浪, 李国基. 一种用于反刍动物口服的金霉素微囊化颗粒及其制备方法: CN109528681A[P]. 2019-03-29. [5] 李子健. 不同生理阶段奶牛瘤胃细菌菌群数量与多样性的比较研究[D]. 呼和浩特: 内蒙古农业大学, 2018. [6] 刘薇, 方国庆, 刘立成, 等. 奶牛瘤胃微生物DNA提取法的研究与优化[J]. 中国畜牧杂志, 2011, 47(13): 71-75. [7] DENMAN S E, MCSWEENEY C S. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen[J]. FEMS Microbiology Ecology, 2006, 58(3): 572-582. doi: 10.1111/j.1574-6941.2006.00190.x
[8] KOIKE S, KOBAYASHI Y. Development and use of competitive PCR assays for the rumen cellulolytic bacteria: Fibrobacter succinogenes, Ruminococcus albusand Ruminococcus flavefaciens[J]. FEMS Microbiology Letters, 2001, 204(2): 361-366. doi: 10.1111/j.1574-6968.2001.tb10911.x
[9] FERNANDO S C, PURVIS H T, NAJAR F Z, et al. Rumen microbial population dynamics during adaptation to a high-grain diet[J]. Applied and Environmental Microbiology, 2010, 76(22): 7482-7490. doi: 10.1128/AEM.00388-10
[10] COBELLIS G, YU Z, FORTE C, et al. Dietary supplementation of Rosmarinus officinalis L. leaves in sheep affects the abundance of rumen methanogens and other microbial populations[J]. Journal of Animal Science and Biotechnology, 2016, 7. doi: 10.1186/s40104-016-0086-8.
[11] 李大彪, 张梅梅, 于永强, 等. 单宁和聚乙二醇对绵羊和山羊瘤胃纤维降解菌数量的影响[J]. 动物营养学报, 2015, 27(2): 596-605. doi: 10.3969/j.issn.1006-267x.2015.02.032 [12] 伍涛, 杨旭, 李书至. 金霉素在畜牧业中的应用概述[J]. 四川畜牧兽医, 2015, 42(10): 35-36. doi: 10.3969/j.issn.1001-8964.2015.10.014 [13] 伍涛, 郭红伟, 李书至. 饲料级金霉素研究进展[J]. 中国动物保健, 2014, 16(12): 18-20. doi: 10.3969/j.issn.1008-4754.2014.12.007 [14] 马立保, 李梦超, 黄敏, 等. 一种用于反刍动物口服的恩诺沙星微囊及其制备方法: CN106176667A[P]. 2016-12-07. [15] 靳茹文, 严根文, 林芝. 一种过瘤胃包被恩诺沙星微丸及其制备方法: CN105106175A[P]. 2015-12-02. [16] TURNER A W, HODGETTS V E. Depression of ruminal digestion in adult sheep by aureomycin[J]. Australian Journal of Agricultural Research, 1952, 3(4): 453-459. doi: 10.1071/AR9520453
[17] LODGE J R, MILES J T, JACOBSON N L, et al. Influence of chlortetracycline on in vitro cellulose digestion by bovine rumen microorganisms[J]. Journal of Dairy Science, 1956, 39(3): 303-311. doi: 10.3168/jds.S0022-0302(56)94749-X
[18] MUNCH-PETERSEN E, ARMSTRONG J. The influence of orally administered oxytetracycline on the rumen bacteria of sheep[J]. The Australian Journal of Experimental Biology and Medical Science, 1958, 36(1): 77-82. doi: 10.1038/icb.1958.9
[19] MAO S Y, ZHANG R Y, WANG D S, et al. Impact of subacute ruminal acidosis (SARA) adaptation on rumen microbiota in dairy cattle using pyrosequencing[J]. Anaerobe, 2013, 24: 12-19. doi: 10.1016/j.anaerobe.2013.08.003
[20] VAREL V H, DEHORITY B A. Ruminal cellulolytic bacteria and protozoa from bison, cattle-bison hybrids, and cattle fed three alfalfa-corn diets[J]. Applied and Environmental Microbiology, 1989, 55(1): 148-153. doi: 10.1128/AEM.55.1.148-153.1989
[21] 杨宏波, 刘红, 占今舜, 等. 不同精粗比颗粒饲料对断奶公犊牛瘤胃发酵参数和微生物的影响[J]. 草业学报, 2015, 24(12): 131-138. doi: 10.11686/cyxb2015022 [22] 林波, 梁辛, 李丽莉, 等. 饲粮精粗比对泌乳水牛瘤胃细菌和甲烷菌区系的影响[J]. 动物营养学报, 2016, 28(10): 3101-3109. doi: 10.3969/j.issn.1006-267x.2016.10.012 [23] 王超, 刘国道. 瘤胃微生物降解纤维素的研究进展[J]. 安徽农业科学, 2007, 35(13): 3771-3772. doi: 10.3969/j.issn.0517-6611.2007.13.002 [24] WINDHAM W R, AKIN D E. Rumen fungi and forage fiber degradation[J]. Applied and Environmental Microbiology, 1984, 48(3): 473-476. doi: 10.1128/AEM.48.3.473-476.1984
[25] HESPELL R B, WOLF R, BOTHAST R J. Fermentation of xylans by Butyrivibrio fibrisolvensand other ruminal bacteria[J]. Applied and Environmental Microbiology, 1987, 53(12): 2849-2853. doi: 10.1128/AEM.53.12.2849-2853.1987
[26] 那仁图雅, 周非帆, 杨金丽, 等. 绵羊瘤胃内纤维降解菌的分离鉴定[J]. 中国畜牧兽医, 2014, 41(5): 142-146. [27] 王卫云, 李大彪, 于永强, 等. 瘤胃4株纤维降解细菌的分离鉴定及其纤维降解特性[J]. 畜牧兽医学报, 2016, 47(11): 2294-2300. doi: 10.11843/j.issn.0366-6964.2016.11.018 [28] 王全军, 朱伟云. 瘤胃微生物及其在饲料工业中的应用前景[J]. 动物科学与动物医学, 2000, 17(3): 64-66. [29] KOIKE S, PAN J, KOBAYASHI Y, et al. Kinetics of in sacco fiber-attachment of representative ruminal cellulolytic bacteria monitored by competitive PCR[J]. Journal of Dairy Science, 2003, 86(4): 1429-1435. doi: 10.3168/jds.S0022-0302(03)73726-6
[30] MICHALET-DOREAU B, FERNANDEZ I, FONTY G. A comparison of enzymatic and molecular approaches to characterize the cellulolytic microbial ecosystems of the rumen and the cecum[J]. Journal of Animal Science, 2002, 80(3): 790-796. doi: 10.2527/2002.803790x



 下载:
下载: