Preparation of monoclonal antibody against N protein of porcine epidemic diarrhea virus and establishment of indirect immuno-fluorescence assay
-
摘要:目的
制备猪流行性腹泻病毒(PEDV) N蛋白单克隆抗体,并建立检测PEDV的间接免疫荧光试验方法。
方法以重组表达的PEDV N蛋白为免疫原,免疫8周龄雌性BALB/c小鼠,分离高抗体效价小鼠的脾细胞,与SP2/0细胞融合。筛选分泌抗PEDV N蛋白单克隆抗体的杂交瘤细胞株。在已经感染PEDV的Vero细胞中,以抗PEDV N蛋白的单克隆抗体为一抗,FITC−羊抗鼠IgG为二抗,建立PEDV的间接免疫荧光检测方法。
结果制备的杂交瘤细胞株可以稳定分泌抗PEDV N蛋白抗体,细胞上清液的ELISA抗体效价在1∶3 200以上,而诱导的小鼠腹水抗体效价在1∶1 000 000以上。将单克隆抗体应用在间接免疫荧光试验时,最适条件为−20 ℃ 80%(φ)丙酮溶液中固定30 min;一抗用PBS缓冲液按体积比1∶1 000稀释,4 ℃条件下过夜孵育;二抗用PBS缓冲液按体积比1∶100稀释,37 ℃条件下孵育1 h。以建立的间接免疫荧光试验方法检测细胞中的猪传染性胃肠炎病毒(TGEV)、猪瘟病毒(CSFV)、猪繁殖与呼吸综合征病毒(PRRSV)、猪伪狂犬病毒(PPRV)、猪肠道α冠状病毒(PEAV)、猪轮状病毒(PoRV)和PEDV,只有PEDV显示阳性,其他病毒均为阴性。
结论制备了抗PEDV N蛋白单克隆抗体,以该抗体为一抗建立检测PEDV的间接免疫荧光试验方法具有良好的特异性,为PEDV的实验室检测及PEDV在培养细胞中的定位和动态分布提供了有效的手段。
Abstract:ObjectiveTo prepare monoclonal antibodies against porcine epidemic diarrhea virus (PEDV) N protein, and develop an indirect immuno-fluorescence assay method used for detecting PEDV.
MethodThe expressed recombinantly PEDV N protein was used as an immunogen and 8-week-old female BALB/c mice were immunized. Then their spleen cells with high antibody titer were isolated and fused with SP2/0 cells. The hybridoma cell lines secreting monoclonal antibodies against PEDV N protein were screened. In Vero cells infected with PEDV, monoclonal antibody of anti-PEDV N protein was used as the primary antibody and FITC-goat-anti-mouse IgG was used as the secondary antibody to develop indirect immuno-fluorescence assay method used for detecting PEDV.
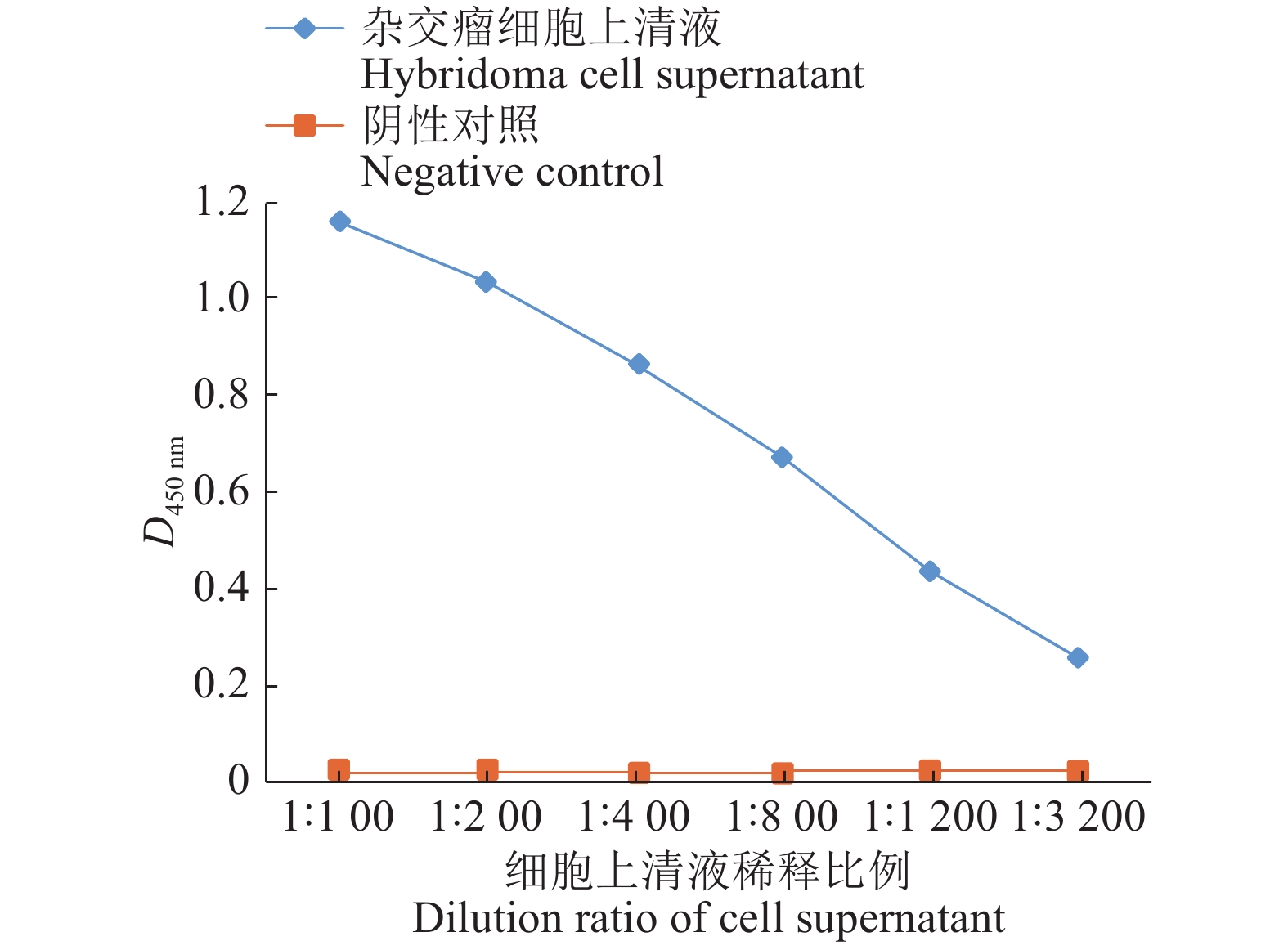
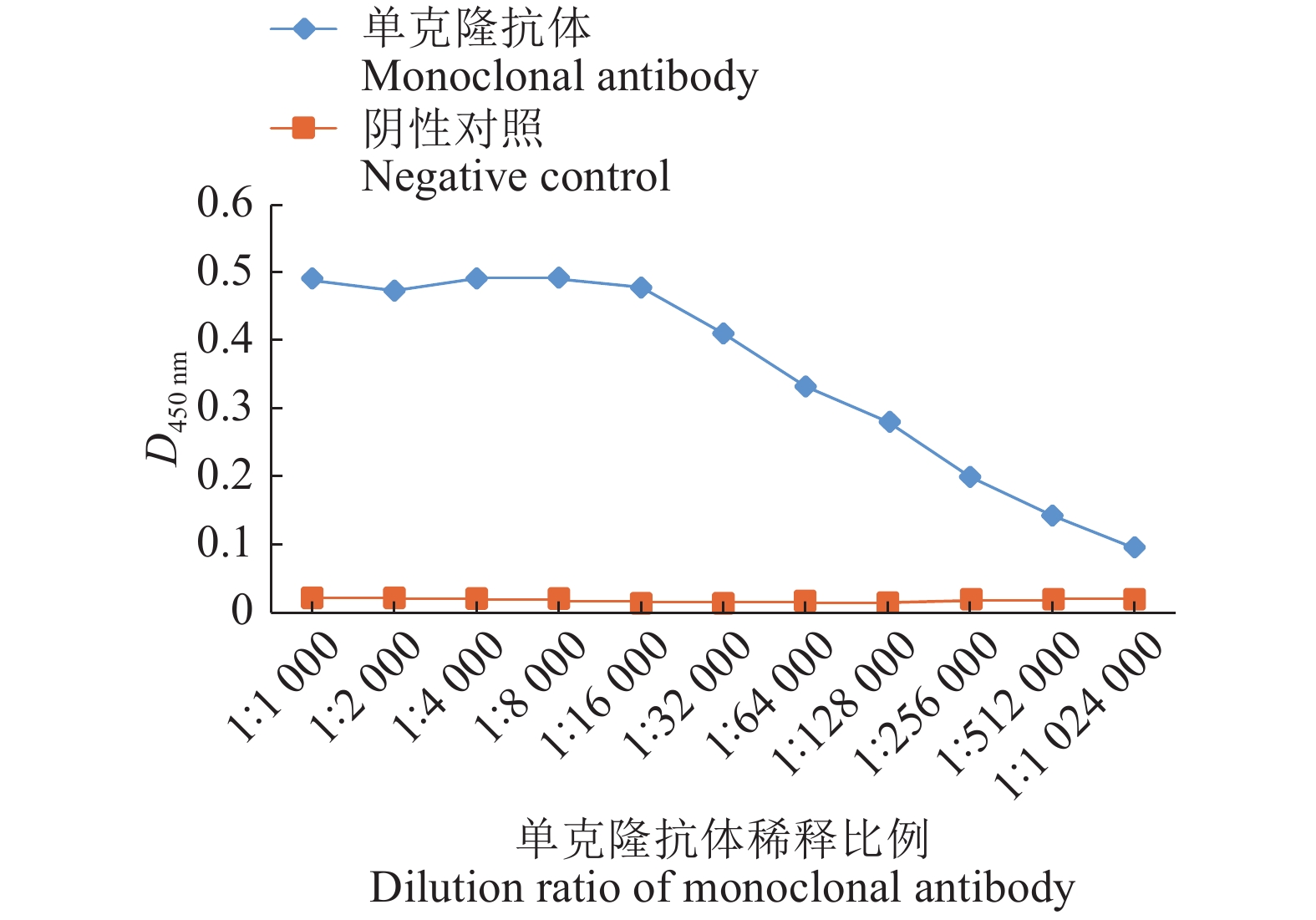
ResultThe prepared hybridoma cell lines could stably secrete anti-PEDV N protein antibodies, ELISA antibody titer in cell supernatant was above 1∶3 200, and in mouse ascites above 1∶1 000 000. While monoclonal antibodies were applied in established indirect immuno-fluorescence assay, the optimal conditions were that cells were fixed with 80% (φ) acetone at −20 ℃ for 30 min; The primary antibody was diluted 1 000 times by PBS buffer solution and incubated at 4 ℃ overnight; The secondary antibody was diluted 100 times by PBS buffer solution and incubated at 37 ℃ for 1 h. Transmissible gastroenteritis virus (TGEV), classical swine fever virus (CSFV), porcine reproductive and respiratory syndrome virus (PRRSV), porcine reproductive virus (PRV), porcine enteric α corone virus (PEAV), porcine rotavirus (PoRV) and PEDV were detected by established indirect immuno-fluorescence assay method, only PEDV showed positive, all the else viruses showed negative.
ConclusionAn anti-PEDV N protein monoclonal antibody is prepared, and the indirect immuno-fluorescence assay method used for detecting PEDV is established with high specificity. It provides an effective method for laboratory detection of PEDV and for localization and dynamic distribution of PEDV in infected cells.
-
猪流行性腹泻(Porcine epidemic diarrhea,PED)是由猪流行性腹泻病毒(Porcine epidemic diarrhea virus,PEDV)引起的一种高度接触性猪肠道传染疾病。PEDV主要引起10日龄以内的仔猪发病,典型的症状为水样腹泻、脱水、呕吐等。自1971年英国首次报道PED后,该病已在世界各地流行[1-4]。2011年以来,我国多个地区暴发了PEDV变异毒株引起的PED[5-8],新生仔猪发病率和死亡率高达90%以上,给我国养猪业造成了巨大损失[9-10]。
PEDV是有囊膜的单股正链RNA病毒,属于冠状病毒科α冠状病毒属的成员,主要结构蛋白包括纤突(Spike,S)蛋白、膜(Membrane,M)蛋白、包膜(Envelope,E)蛋白和核衣壳(Nucleocapsid,N)蛋白[11]。每种蛋白都在病毒的繁殖中发挥重要作用。N蛋白高度保守,在病毒的结构蛋白中所占比例最大[12-13],也是病毒复制过程中表达量最大的一种碱性结构蛋白,对诱导细胞免疫至关重要。与S蛋白一样,N蛋白也是早期准确检测PEDV感染的靶蛋白[14-15]。N蛋白还是一种重要的抗原,在病毒感染机体的早期刺激机体产生体液免疫,诱导机体产生大量抗N蛋白抗体,普遍用于针对PEDV的血清学检测。
本研究基于N蛋白的特点,以原核表达的方法制备重组N蛋白,制备抗N蛋白单克隆抗体,并使用该抗体建立PEDV间接免疫荧光试验的抗体检测方法,为PEDV的实验室检测及PEDV在感染细胞中的定位和动态分布研究提供有效手段。
1. 材料与方法
1.1 菌种、毒株、细胞系
原核表达载体pET-32a(+),PEDV(CH/GDZH02/1401,简称ZH02),猪传染性胃肠炎病毒(Transmissible gastroenteritis virus,TGEV)、猪伪狂犬病毒(Porcine pseudorabies virus,PPRV)、猪瘟病毒(Classical swine fever virus,CSFV)、猪繁殖与呼吸综合征病毒(Porcine reproductive and respiratory syndrome virus,PRRSV)、Vero细胞、PEDV抗体均由华南农业大学兽医学院微生物学与免疫学教研室保存。SP2/0骨髓瘤细胞、猪肠道α冠状病毒(Porcine enteric α corone virus,PEAV)、猪轮状病毒(Porcine rotavirus,PoRV)分别由华南农业大学琚春梅博士、马静云教授和江苏农业科学院兽医研究所何孔旺研究员惠赠。
1.2 主要试剂
50×HAT(次黄嘌呤−氨甲碟呤−胸腺嘧啶核苷)、HT(次黄嘌呤−胸腺嘧啶核苷)、弗氏佐剂和弗氏不完全佐剂均为Sigma公司产品。HRP−羊抗鼠IgG为碧云天公司产品。FITC−羊抗鼠IgG为博奥森公司产品。
1.3 试验动物
SPF BALB/c 6~8周龄雌性小鼠购自南方医科大学实验动物中心。
1.4 PEDV N蛋白重组质粒的构建、表达与鉴定
根据ZH02毒株(GenBank注册号为KR153326)基因组序列,设计1对引物:PEDV-N-F:5′-CGGCATATGATGGCTTCTGTCAGTTTTCA-3′,PEDV-N-R:5′-CCGCTCGAGATTTCCTGTGTCGAAGATCTC-3′。提取病毒RNA,以PEDV-N-F和PEDV-N-R为引物通过RT-PCR扩增 N 基因,与pET-32(a)连接,构建PEDV N 基因重组表达质粒,命名为pET-N。将构建的重组表达载体转化大肠埃希菌Transetta(DE3)进行N蛋白的表达和纯化,随后将纯化好的蛋白进行Western blot鉴定。
1.5 动物免疫
6只8周龄雌性BALB/c小鼠饲养于华南农业大学动物实验中心。300 μg的PEDV N蛋白与等体积(500 μL)弗氏完全佐剂一起乳化制备免疫原,皮下注射小鼠。一次免疫后第15天和第29天,用等体积(500 μL)弗氏不完全佐剂乳化300 μg抗原分别进行二次免疫和三次免疫。三次免疫后第10天检测小鼠抗N蛋白抗体效价,当抗体效价达到1∶100 000后,于融合前3 d腹腔注射非乳化抗原150 μg加强免疫一次。
1.6 抗体滴度的测定
在96孔酶标反应板中,每孔加入100 μL纯化后的N蛋白(ρ为8 μg/mL),37 ℃条件下温育1 h,然后4 ℃条件下过夜。弃去包被液,用PBST缓冲液洗涤3次。每孔加入100 μL封闭液,37 ℃恒温箱中封闭2 h。弃去封闭液,用PBST缓冲液洗涤3次,加入用PBS缓冲液倍比稀释的血清或细胞上清液,置于37 ℃条件下温育70 min。弃去孵育液,用PBST缓冲液洗涤3次,加入100 μL HRP−羊抗鼠IgG(用PBS缓冲液按体积比1︰10 000稀释)的二抗,置于37 ℃条件下温育40 min。弃去孵育液,用PBST缓冲液洗涤3次,加入100 μLTMB底物,37 ℃恒温箱避光显色5~10 min,加入50 μL终止液终止反应,用酶标仪测定D450 nm,计算抗体滴度。
1.7 杂交瘤细胞的筛选
以聚乙二醇(PEG)1450为融合剂将获得的阳性小鼠脾细胞和SP2/0骨髓瘤细胞融合,融合后第10天按照“1.6”的方法检测细胞上清液,筛选抗体呈阳性细胞孔。之后对阳性孔中的杂交瘤细胞进行亚克隆,每亚克隆1次需要对细胞上清液进行ELISA检测,直到获得单个杂交瘤细胞株。亚克隆成功后,将杂交瘤细胞株置于液氮中保存。
1.8 单克隆抗体的制备、检测和鉴定
1.8.1 制备
取生长状态良好、抗体分泌能力强的杂交瘤细胞株通过体内诱生法制备腹水。将弗氏不完全佐剂按每只500 μL的剂量腹腔注射雌性BALB/c小鼠,致敏7 d备用。从细胞培养瓶中吹下杂交瘤细胞,调整细胞数至1×106/mL,用1640培养液洗涤2~3次后腹腔注射经弗氏不完全佐剂致敏的小鼠。10~15 d后,小鼠腹部明显膨大时抽取腹水,经4 000 r/min离心20 min,收集上清液,ELISA测定抗体效价后分装冻存于−80 ℃。
1.8.2 单克隆抗体的特异性检测
为验证获得的单克隆抗体的特异性,以TGEV、PPRV、CSFV、PRRSV为抗原包被酶标板,进行间接ELISA检测。
1.8.3 单克隆抗体的Western blot鉴定
将表达的N蛋白经SDS-PAGE电泳后转移到PVDF膜上,再将膜放入50 g/L脱脂牛乳溶液中封闭,置于37 ℃条件下孵育2 h;使用PBST缓冲液洗涤4次,将制备的腹水用PBS缓冲液按体积比1∶5 000稀释作为一抗,置于4 ℃条件下过夜孵育;使用PBST缓冲液洗涤4次,HRP−羊抗鼠IgG用PBS缓冲液按体积比1∶5 000稀释后作为二抗,置于37 ℃条件下中孵育1 h;使用PBST缓冲液洗涤4次,加入DAB显色。
1.9 间接免疫荧光试验
1.9.1 试验方法的建立
待96孔板的Vero细胞长成单层后,接种PEDV。20 h后,弃培养液,每孔加入150 μL预冷的80%(φ)丙酮溶液,−20 ℃条件下固定30 min。随后每孔用灭菌的PBS缓冲液洗涤3次,再于每孔加入50 μL用PBS缓冲液稀释的一抗N蛋白单克隆抗体(N蛋白单克隆抗体与PBS缓冲液的体积比为1∶200),37 ℃条件下孵育2 h。用灭菌的PBS缓冲液洗涤3次,每孔加入50 μL用PBS缓冲液稀释的二抗FITC−羊抗鼠IgG(FITC−羊抗鼠IgG与PBS缓冲液的体积比为1∶ 100),在37 ℃条件下孵育1 h。用灭菌的PBS缓冲液洗涤3次,最后每孔加入100 μL灭菌的PBS缓冲液,置于荧光倒置显微镜下观察。
1.9.2 试验方法的优化
本试验主要对一抗工作浓度、二抗工作浓度和孵育时间进行了优化。一抗工作浓度:分别按体积比1∶100、1∶200、1∶500、1∶1 000、1∶2 000稀释;二抗工作浓度:分别按体积比1∶100、1∶200、1∶500、1∶800稀释;一抗孵育时间:4 ℃条件下过夜孵育、37 ℃条件下孵育2 h。镜检观察结果。
1.9.3 特异性试验
在长成单层的Vero细胞中分别接种CSFV、PRRSV、TGEV、PPRV、PEAV、PoRV和PEDV,同时设立不接病毒的正常细胞为对照。接种24 h后,用“1.12”建立的间接免疫荧光方法进行检测。
1.9.4 无一抗对照试验
用PBS缓冲液替代一抗进行间接免疫荧光试验,观察二抗是否有特异性反应,同时设立阳性对照。
1.9.5 重复性试验
取同批次和不同批次的Vero细胞接种病毒,按“1.9.2”建立的间接免疫荧光方法进行试验,检测本方法是否具有重复性。
1.9.6 样品自发光对照试验
将未接种病毒的细胞板和接种病毒的细胞板弃去培养基后,每孔加入100 μL灭菌的PBS缓冲液,然后置于荧光显微镜下观察是否存在自发荧光现象,同时设阳性对照。
1.9.7 敏感性试验
将PEDV病毒液用不完全培养基DMEM按照10倍倍比梯度(1∶10、1∶100、1∶1 000、1∶10 000、1∶100 000、1∶1 000 000)进行稀释,按照“1.9.2”建立的间接免疫荧光试验方法进行试验,以出现明显荧光的最大稀释倍数的病毒稀释度作为检测的最高灵敏度。
2. 结果与分析
2.1 PEDV N蛋白重组质粒的构建
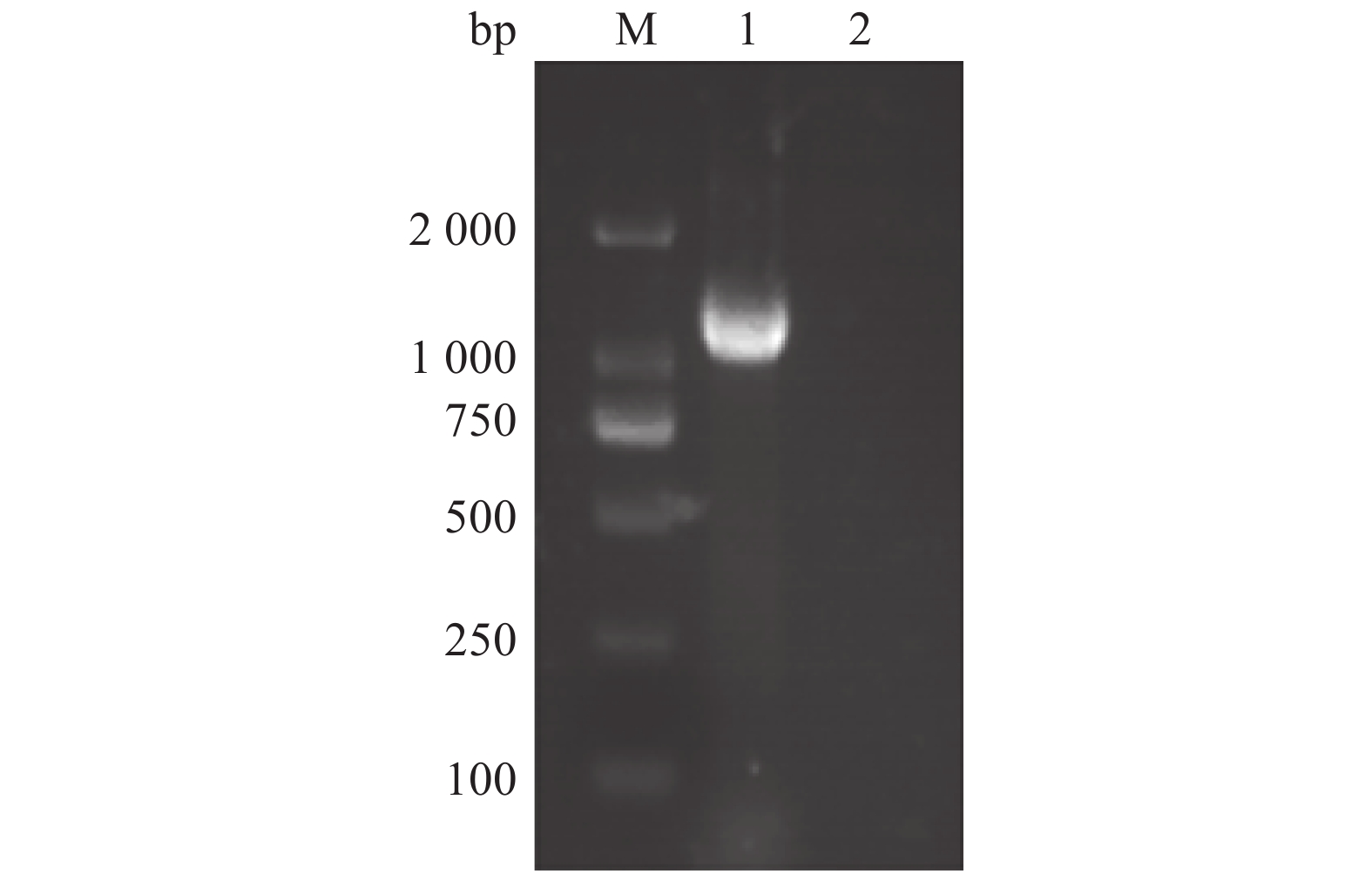
以提取的PEDV核酸为模板,通过RT-PCR扩增 N 基因,再插入pET质粒的多克隆位点,构建表达重组质粒pET-N。再以该重组子菌液为模板,用PEDV-N-F、PEDV-N-R为引物进行RT-PCR扩增。结果显示,扩增片段为1 400 bp左右,与预期结果一致(图1)。
2.2 PEDV N蛋白的表达、纯化与鉴定
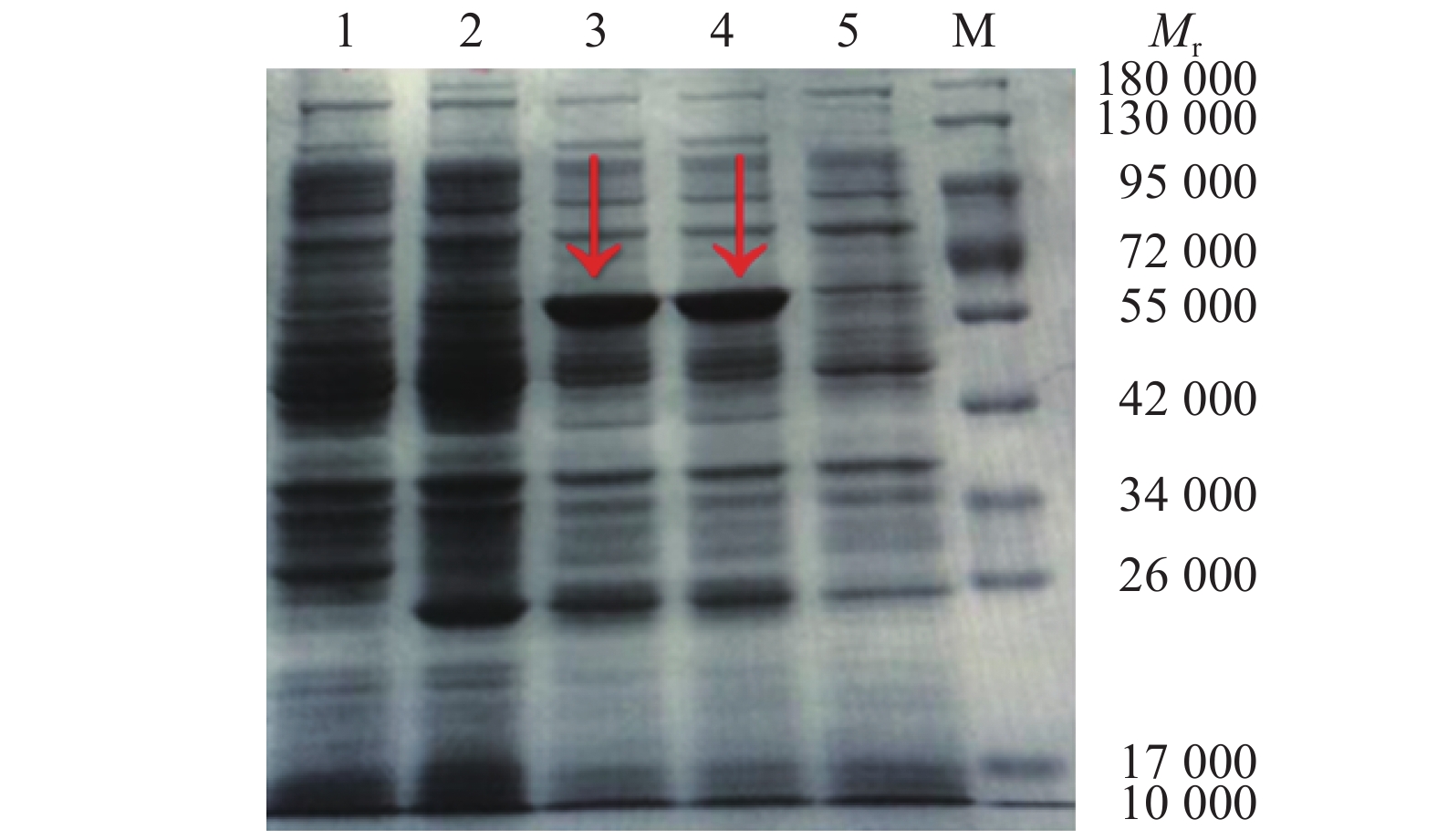
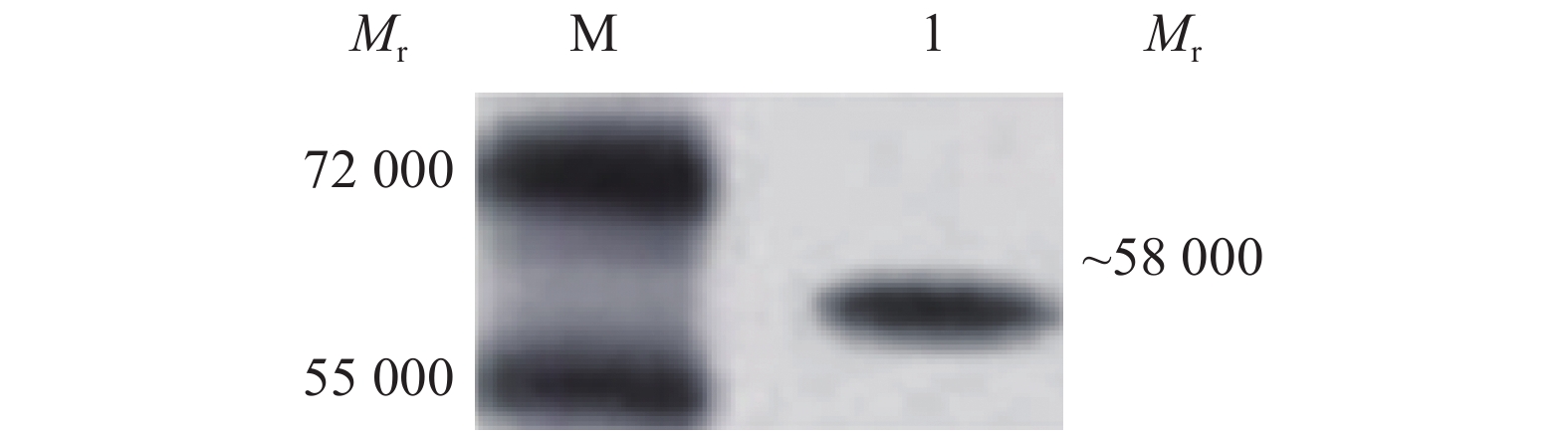
重组质粒pET-N转化进表达宿主菌Transetta(DE3),经IPTG诱导后进行SDS-PAGE分析,重组子pET-N表达产物中出现相对分子质量为58 000的特异目的蛋白条带,与预期大小相符。空载体宿主菌表达产物无特异性条带出现,未经诱导的重组子表达产物中出现微弱的特异性条带(图2)。纯化的N蛋白转膜后,以抗PEDV的抗体染色,发现相对分子质量为58 000的特异性条带(图3)。
![图 2 PET-N表达产物的SDS-PAGE]() 图 2 PET-N表达产物的SDS-PAGEMr: 相对分子质量;M:蛋白marker;1:空载体宿主菌诱导产物;2、5:未诱导的含PET-N宿主菌菌株;3、4:pET-N诱导产物Figure 2. SDS-PAGE of expressed product PET-NMr: Relative molecular mass; M: Protein marker; 1: Induction products of empty vector host bacteria; 2, 5: Uninduced host bacteria strain with PET-N; 3, 4: pET-N induced products
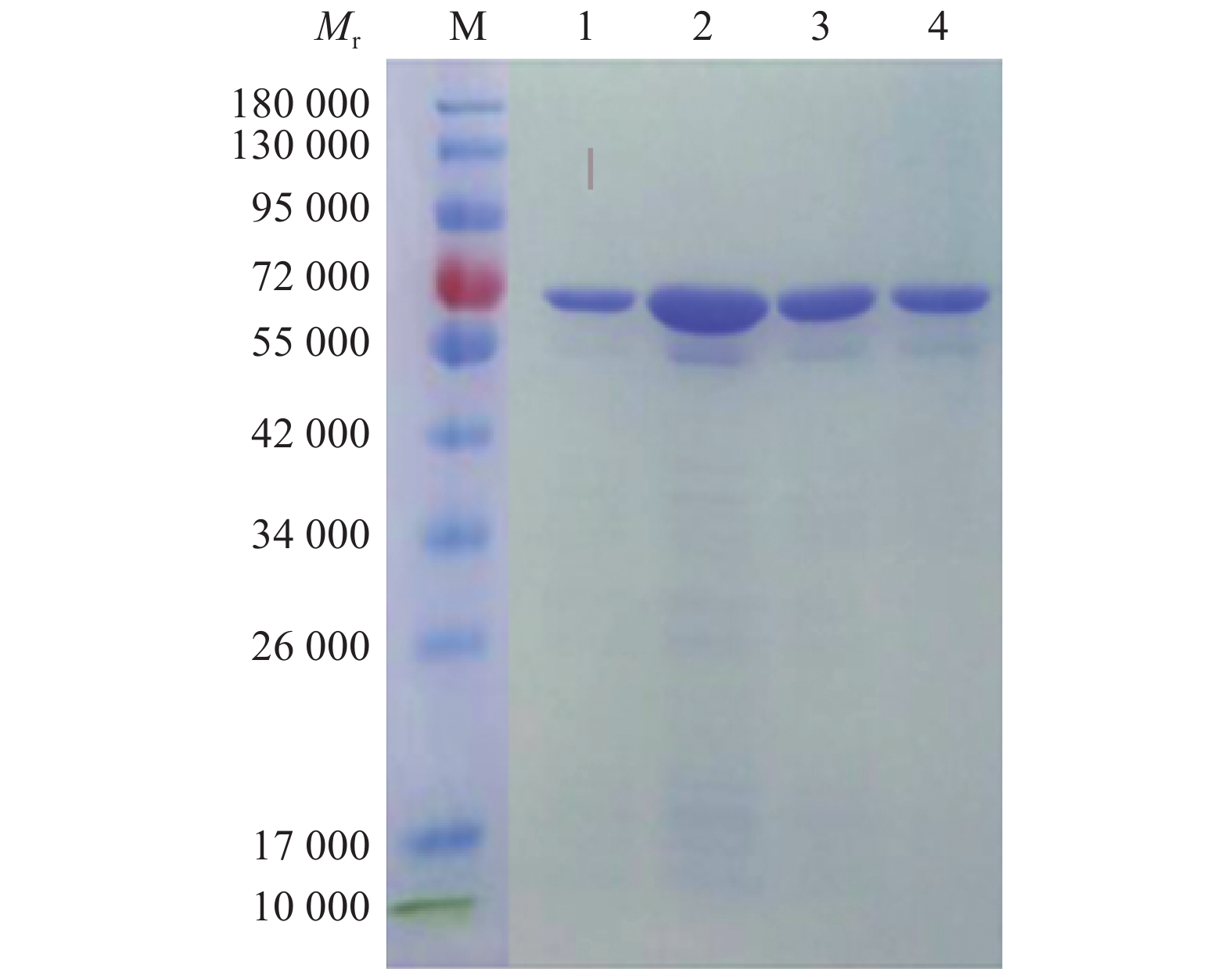
图 2 PET-N表达产物的SDS-PAGEMr: 相对分子质量;M:蛋白marker;1:空载体宿主菌诱导产物;2、5:未诱导的含PET-N宿主菌菌株;3、4:pET-N诱导产物Figure 2. SDS-PAGE of expressed product PET-NMr: Relative molecular mass; M: Protein marker; 1: Induction products of empty vector host bacteria; 2, 5: Uninduced host bacteria strain with PET-N; 3, 4: pET-N induced productspET-N重组子在优化条件下诱导表达后,目的蛋白经重力流Ni柱层析纯化,收集不同浓度咪唑洗脱的蛋白液,用透析膜脱盐处理后进行SDS-PAGE,用考马斯亮蓝染色,脱色液脱色。结果(图4)显示,纯化的蛋白相对分子质量约58 000,且目的蛋白纯度较高,根据结果选用300 mmol/L咪唑洗脱的蛋白,用BCA试剂盒测定蛋白质量浓度为450 μg/mL。
![图 4 经不同浓度咪唑洗脱的纯化产物的SDS-PAGE]() 图 4 经不同浓度咪唑洗脱的纯化产物的SDS-PAGEMr:相对分子质量;M:蛋白 marker;1:300 mmol·L−1咪唑洗脱的蛋白液;2:500 mmol·L−1咪唑洗脱的蛋白液;3、4:400 mmol·L−1咪唑洗脱的蛋白液Figure 4. SDS-PAGE of purified product eluted by different concentrations of imidazoleMr: Relative molecular mass; M: Protein marker; 1: 300 mmol·L−1 imidazole eluted protein solution; 2: 500 mmol·L−1 imidazole eluted protein solution; 3, 4: 400 mmol·L−1 imidazole eluted protein solution
图 4 经不同浓度咪唑洗脱的纯化产物的SDS-PAGEMr:相对分子质量;M:蛋白 marker;1:300 mmol·L−1咪唑洗脱的蛋白液;2:500 mmol·L−1咪唑洗脱的蛋白液;3、4:400 mmol·L−1咪唑洗脱的蛋白液Figure 4. SDS-PAGE of purified product eluted by different concentrations of imidazoleMr: Relative molecular mass; M: Protein marker; 1: 300 mmol·L−1 imidazole eluted protein solution; 2: 500 mmol·L−1 imidazole eluted protein solution; 3, 4: 400 mmol·L−1 imidazole eluted protein solution2.3 抗体效价
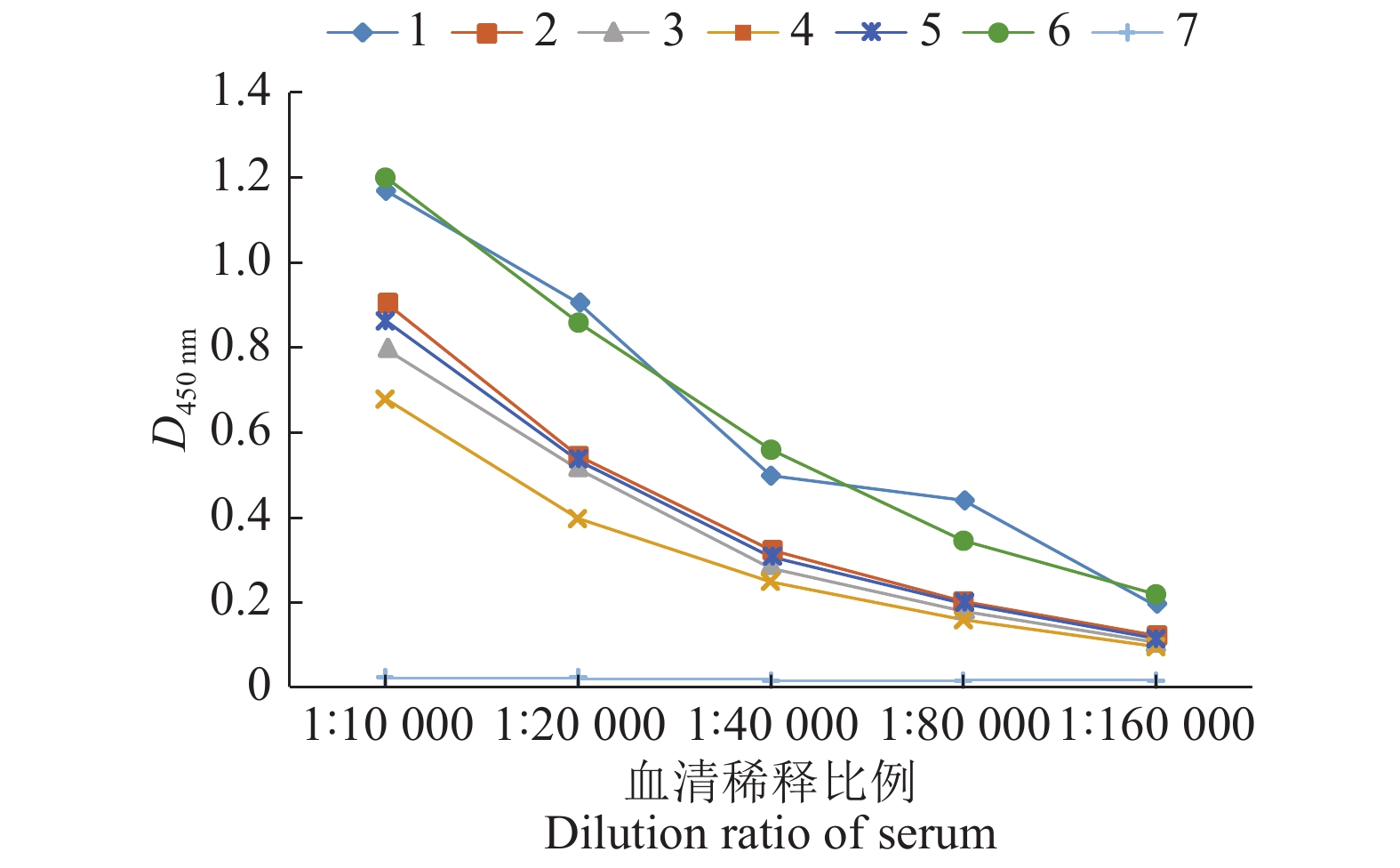
小鼠经3次免疫后,用间接ELISA测定各组小鼠的抗体效价,其中2~5号小鼠的抗体水平较低,1号、6号小鼠的抗体水平较高,且6号小鼠的ELISA结果较为稳定,结果见图5。后续试验选用6号小鼠进行。
经过2次亚克隆,获得1株分泌特异性单抗的杂交瘤细胞,命名为13。收集杂交瘤细胞上清液,用PBS缓冲液倍比稀释,用间接ELISA检测杂交瘤细胞上清液效价。将杂交瘤细胞上清液稀释3 200倍后仍为阳性,结果见图6。
将杂交瘤细胞13接种小鼠腹腔制备单克隆抗体。抽取腹水并测定其抗体效价,制备的PEDV N蛋白单克隆抗体用PBS缓冲液经过1 024 000倍稀释后仍为阳性,证明该单抗抗体水平较高,结果见图7。
2.4 特异性检测和Western blot 鉴定结果
单克隆抗体的特异性检测结果见表1。以TGEV、PPRV、CSFV、PRRSV和PEDV为抗原包被8 μg/mL的纯化N蛋白,将腹水用PBS缓冲液按体积比1∶10 000稀释作为一抗,采用间接ELISA法进行检测,结果显示,包被不同稀释比例的TGEV、PPRV、CSFV、PRRSV均呈阴性反应,只有PEDV呈阳性,各稀释比例下的D450 nm稳定。
表 1 间接ELISA法检测不同稀释比例病毒的抗体特异性Table 1. Detection of antibody specificity of viruses with different dilution ratios by indirect ELISA病毒名称1)
Virus nameD450 nm 1∶1 1∶100 1∶1 000 1∶3 000 1∶5 000 1∶10 000 1∶50 000 1∶100 000 TGEV 0.022 0.019 0.026 0.021 0.022 0.018 0.024 0.026 PRV 0.020 0.020 0.023 0.023 0.026 0.022 0.023 0.021 CSFV 0.018 0.017 0.019 0.020 0.017 0.021 0.020 0.016 PRRSV 0.018 0.020 0.023 0.021 0.017 0.020 0.022 0.018 PEDV 0.489 0.474 0.492 0.491 0.479 0.472 0.468 0.492 1)TGEV、PPRV、CSFV、PRRSV、PEDV 分别代表猪传染性胃肠炎病毒、猪伪狂犬病毒、猪瘟病毒、猪繁殖与呼吸综合征病毒、猪流行性腹泻病毒
1) TGEV, PPRV, CSFV, PRRSV and PEDV N indicate transmissible gastroenteritis virus, classical swine fever virus, porcine reproductive and respiratory syndrome virus and porcine epidemic diarrhea virus将纯化的N蛋白转到PVDF膜后,用脱脂牛乳溶液在37 ℃条件下封闭3 h,将收集并处理好的小鼠腹水用PBS缓冲液按照体积比1∶5 000稀释作为一抗,4 ℃条件下过夜孵育,HRP−羊抗鼠IgG用PBS缓冲液按体积比 1∶5 000稀释作为二抗,37 ℃孵育1 h。进行Western blot分析,在相对分子质量为58 000处有明显的特异性条带,结果见图8。
2.5 最适工价浓度
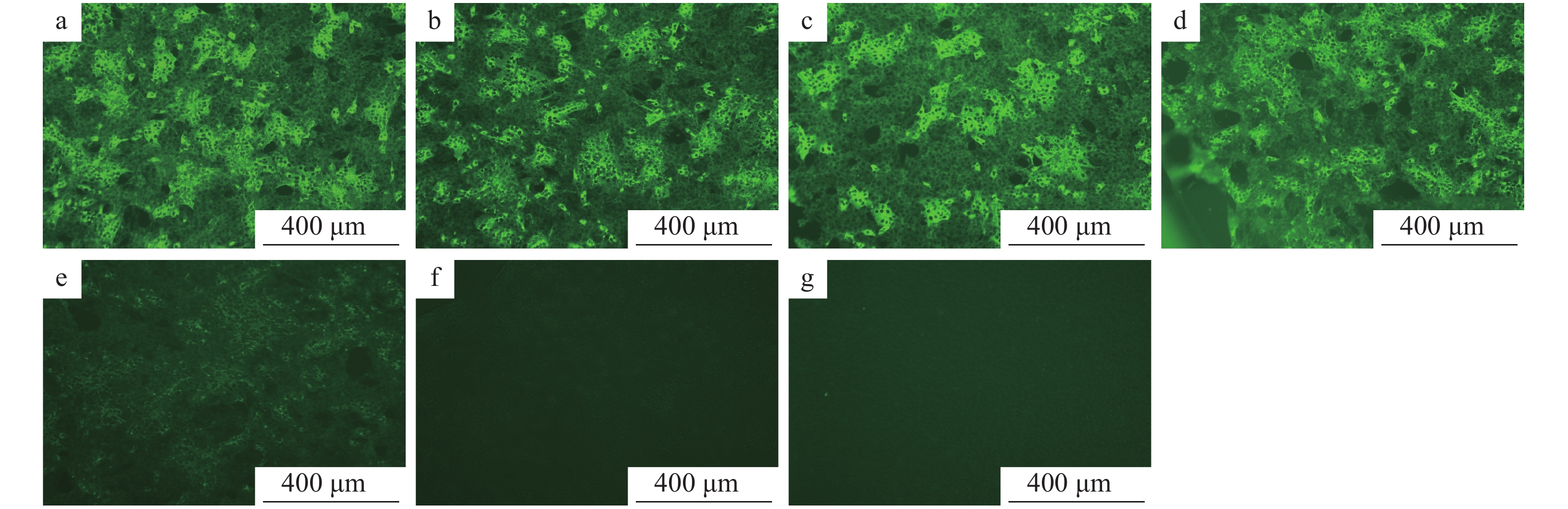
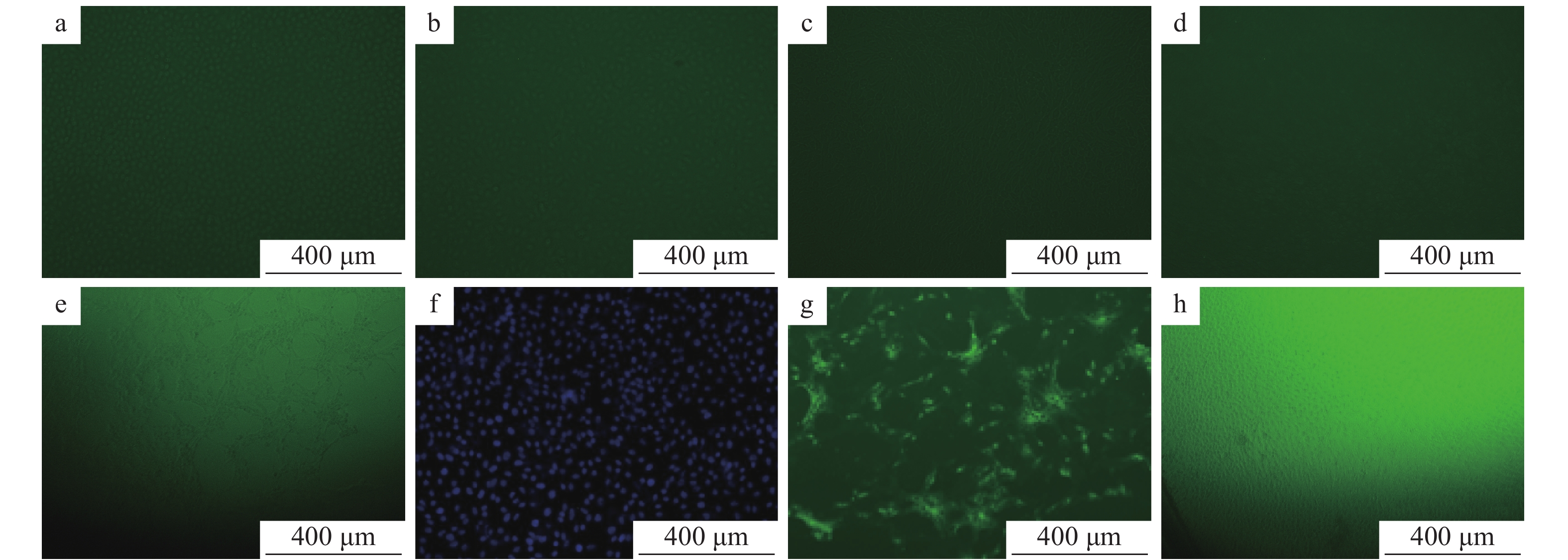
N蛋白单克隆抗体最适工作浓度结果见图9。在已接种PEDV的细胞培养板中,加入用PBS缓冲液倍比稀释的N蛋白单克隆抗体、用PBS缓冲液按体积比1∶100稀释的FITC−羊抗鼠IgG,同时设阴性及空白对照,然后在荧光显微镜下观察。结果显示,阴性血清及空白对照均无肉眼可见的特异性荧光,N蛋白单克隆抗体在稀释比例为1∶1 000时荧光班点最清晰。
![图 9 不同稀释度N蛋白单克隆抗体的间接免疫荧光试验结果]() 图 9 不同稀释度N蛋白单克隆抗体的间接免疫荧光试验结果a、b、c、d、e的稀释比例分别为 1∶100、1∶200、1∶500、1∶1 000、1∶2 000;f:阴性血清;g:空白对照Figure 9. Indirect immuno-fluorescenece assay results of N protein monoclonal antibodies at different dilution ratiosThe dilution ratios of a,b,c,d and e are 1∶100, 1∶200, 1∶500, 1∶1 000 and 1∶2 000 respectively;f:Negative serum;g:Blank control
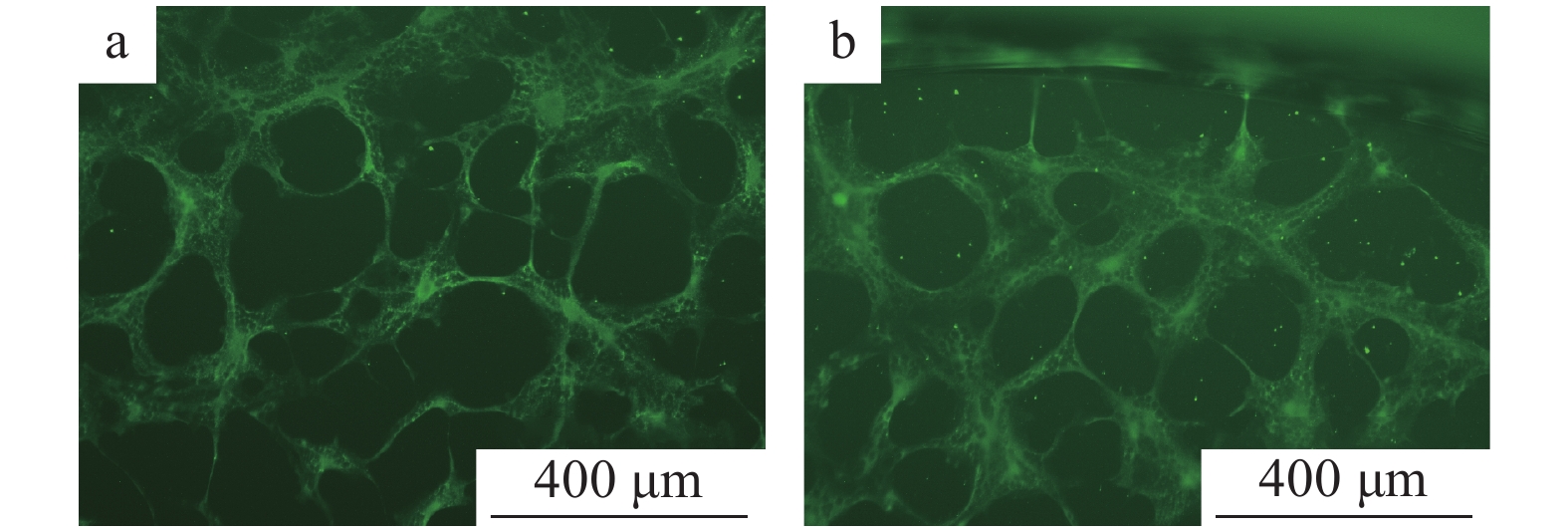
图 9 不同稀释度N蛋白单克隆抗体的间接免疫荧光试验结果a、b、c、d、e的稀释比例分别为 1∶100、1∶200、1∶500、1∶1 000、1∶2 000;f:阴性血清;g:空白对照Figure 9. Indirect immuno-fluorescenece assay results of N protein monoclonal antibodies at different dilution ratiosThe dilution ratios of a,b,c,d and e are 1∶100, 1∶200, 1∶500, 1∶1 000 and 1∶2 000 respectively;f:Negative serum;g:Blank control在已接种PEDV的细胞培养板中加入用PBS缓冲液倍比稀释的FITC−羊抗鼠IgG和用PBS缓冲液按体积比1∶1 000稀释的N蛋白单抗,同时设样本自发荧光对照及阴性对照,在荧光显微镜下观察。结果显示,样品自发荧光对照及阴性对照均无肉眼可见的特异性荧光,而FITC−羊抗鼠IgG的稀释度在1∶100时荧光斑点最清晰(图10)。
2.6 N蛋白单克隆抗体孵育时间
N蛋白单克隆抗体4 ℃条件下过夜孵育和37 ℃条件下孵育2 h,均能观察到亮度无明显差别的绿色荧光(图11)。
2.7 间接免疫荧光试验结果
2.7.1 特异性试验
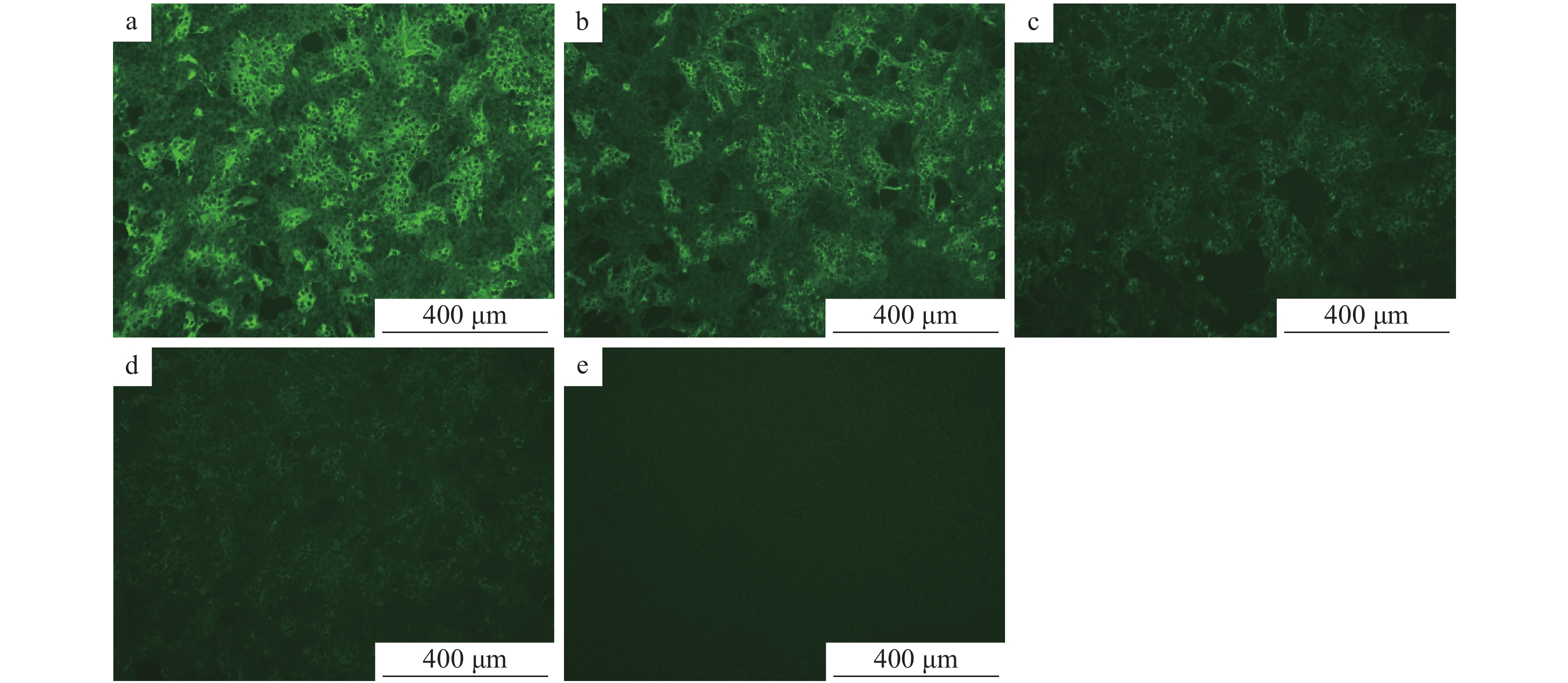
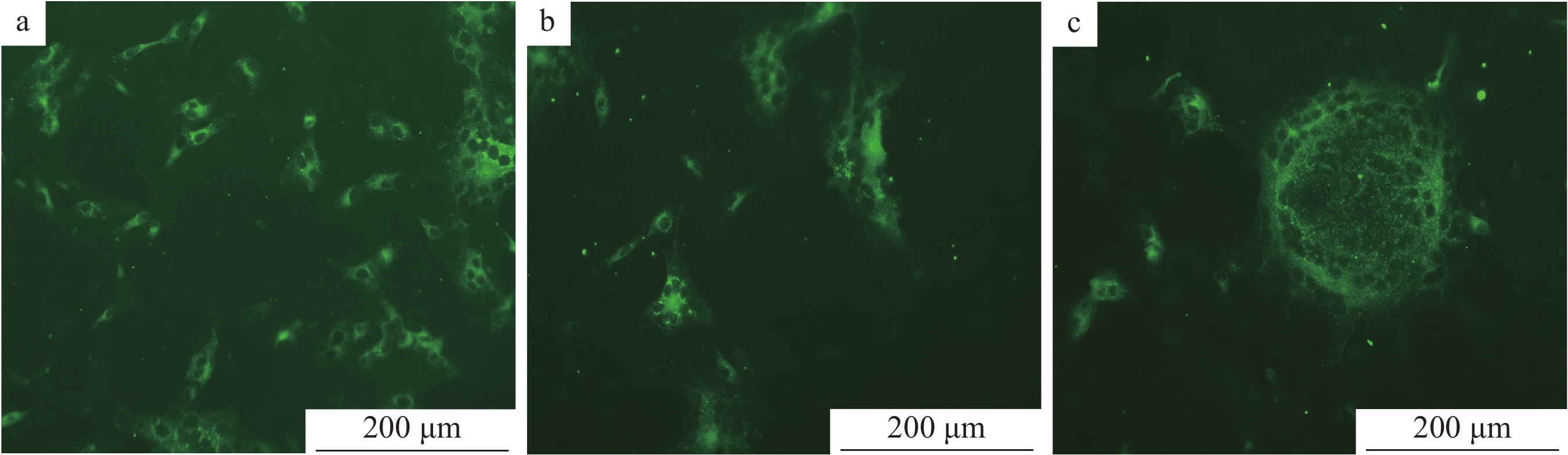
在已接种TGEV、CSFV、PRRSV、PRV、PEAV、PoRV、PEDV的细胞培养板中,加入1∶1 000稀释的PEDV N蛋白单抗、1∶100稀释的FITC–羊抗鼠–IgG,同时设立PEDV阳性对照和正常细胞的阴性对照,然后置于荧光显微镜和激光共聚焦显微镜下观察。结果表明,除接种PEDV的细胞有明显的特异性荧光显色外,接种TGEV、CSFV、PRRSV、PRV、PEAV、PoRV的细胞均无特异性荧光,说明建立的PEDV间接免疫荧光检测方法具有较强的特异性(图12)。
![图 12 间接免疫荧光特异性试验结果]() 图 12 间接免疫荧光特异性试验结果a:猪瘟病毒;b:猪繁殖与呼吸综合征病毒;c:猪伪狂犬病毒;d:猪传染性胃肠炎病毒;e:猪肠道α冠状病毒;f:猪轮状病毒;g:阳性对照;h:阴性对照Figure 12. Specific test results of indirect immuno-fluorescenece assaya: Classical swine fever virus; b: Porcine reproductive and respiratory syndrome virus; c: Porcine pseudorabies virus; d: Transmissible gastroenteritis virus; e: Porcine enteric α corone virus; f: Porcine rotavirus; g: Positive control; h: Negative control
图 12 间接免疫荧光特异性试验结果a:猪瘟病毒;b:猪繁殖与呼吸综合征病毒;c:猪伪狂犬病毒;d:猪传染性胃肠炎病毒;e:猪肠道α冠状病毒;f:猪轮状病毒;g:阳性对照;h:阴性对照Figure 12. Specific test results of indirect immuno-fluorescenece assaya: Classical swine fever virus; b: Porcine reproductive and respiratory syndrome virus; c: Porcine pseudorabies virus; d: Transmissible gastroenteritis virus; e: Porcine enteric α corone virus; f: Porcine rotavirus; g: Positive control; h: Negative control2.7.2 重复性试验
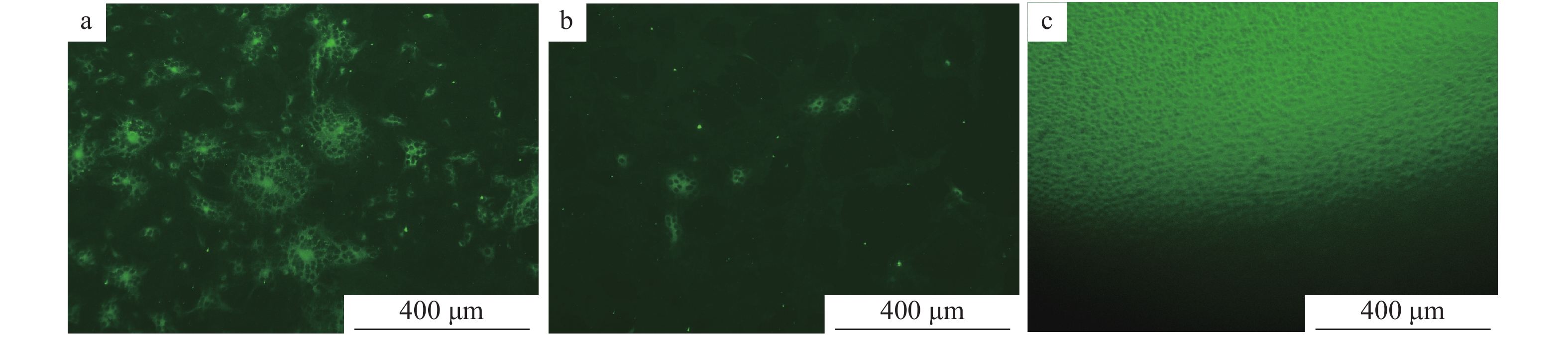
取同批次(图13a、13b)和不同批次(图13c)的Vero细胞接种病毒,采用间接免疫荧光法进行试验,结果显示荧光强度一致,说明重复性好。
2.7.3 敏感性试验
在已接种不同稀释度PEDV病毒液的细胞培养板中,加入用PBS缓冲液按体积比1∶1 000稀释的PEDV N蛋白单克隆抗体、用PBS缓冲液按体积比1∶100稀释的FITC−羊抗鼠IgG,同时设正常细胞为空白对照,然后置荧光显微镜下观察,结果表明,将PEDV病毒液稀释到100 000,在荧光显微镜下仍可观察到典型的特异性绿色荧光(图14)。
![图 14 间接免疫荧光试验敏感性试验结果]() 图 14 间接免疫荧光试验敏感性试验结果a:PEDV病毒液用不完全培养基DMEM按1∶10稀释;b:PEDV病毒液用不完全培养基DMEM按1∶100 000稀释;c:空白对照Figure 14. Sensitivity test results of indirect immuno-fluorescenece assaya:PEDV virus solution is diluted by incomplete medium DMEM at 1∶10;b:PEDV virus solution is diluted by incomplete medium DMEM at 1∶100 000;c:Blank control
图 14 间接免疫荧光试验敏感性试验结果a:PEDV病毒液用不完全培养基DMEM按1∶10稀释;b:PEDV病毒液用不完全培养基DMEM按1∶100 000稀释;c:空白对照Figure 14. Sensitivity test results of indirect immuno-fluorescenece assaya:PEDV virus solution is diluted by incomplete medium DMEM at 1∶10;b:PEDV virus solution is diluted by incomplete medium DMEM at 1∶100 000;c:Blank control3. 讨论与结论
我国养殖业中PEDV感染十分普遍[16],3~10日龄的仔猪最为易感,病死率为80%~100%[17],给我国养猪业造成严重损失[9-10]。在临诊症状和病理变化上,除与猪传染性胃肠炎十分相似外,PED还与PEAV、PoRV、致病性大肠埃希菌引起的病征相似。因此,建立一种PED的实验室诊断方法具有重要实践意义。
本研究利用原核表达系统表达PEDV N蛋白。相比其他表达系统,原核表达系统具有操作简单、价格便宜、表达量大等优点。在单克隆抗体的制备过程中,蛋白的纯度要求高,本研究利用PET-32a(+)载体上自带的HIS标签与Ni柱反应,经考马斯亮蓝染色证明获得了高纯度的目的蛋白,用此蛋白作为免疫原免疫小鼠,小鼠的效价达到1∶80 000以上,证明该目的蛋白具有很好的免疫原性。
PEAV、PoRV、TGEV感染猪只后,临诊症状与PEDV相似,并且TGEV、PoRV接种Vero细胞后产生的细胞病变和PEDV也相似;因此,仅仅依靠临诊症状和细胞病变难以鉴别诊断。本研究建立的免疫荧光试验检测方法经过特异性试验的验证,除了与PEDV有特异性反应可以观察到明显的绿色荧光外,与其他的病原没有交叉反应。
综上所述,本研究利用大肠埃希菌原核表达系统成功构建PET-N重组质粒,表达了PEDV N蛋白,制备了PEDV N单克隆抗体,并利用此抗体建立了检测PEDV的间接免疫荧光试验方法,为PEDV的流行病学调查、临床诊断和疫情控制提供了技术支撑。
-
图 2 PET-N表达产物的SDS-PAGE
Mr: 相对分子质量;M:蛋白marker;1:空载体宿主菌诱导产物;2、5:未诱导的含PET-N宿主菌菌株;3、4:pET-N诱导产物
Figure 2. SDS-PAGE of expressed product PET-N
Mr: Relative molecular mass; M: Protein marker; 1: Induction products of empty vector host bacteria; 2, 5: Uninduced host bacteria strain with PET-N; 3, 4: pET-N induced products
图 4 经不同浓度咪唑洗脱的纯化产物的SDS-PAGE
Mr:相对分子质量;M:蛋白 marker;1:300 mmol·L−1咪唑洗脱的蛋白液;2:500 mmol·L−1咪唑洗脱的蛋白液;3、4:400 mmol·L−1咪唑洗脱的蛋白液
Figure 4. SDS-PAGE of purified product eluted by different concentrations of imidazole
Mr: Relative molecular mass; M: Protein marker; 1: 300 mmol·L−1 imidazole eluted protein solution; 2: 500 mmol·L−1 imidazole eluted protein solution; 3, 4: 400 mmol·L−1 imidazole eluted protein solution
图 9 不同稀释度N蛋白单克隆抗体的间接免疫荧光试验结果
a、b、c、d、e的稀释比例分别为 1∶100、1∶200、1∶500、1∶1 000、1∶2 000;f:阴性血清;g:空白对照
Figure 9. Indirect immuno-fluorescenece assay results of N protein monoclonal antibodies at different dilution ratios
The dilution ratios of a,b,c,d and e are 1∶100, 1∶200, 1∶500, 1∶1 000 and 1∶2 000 respectively;f:Negative serum;g:Blank control
图 12 间接免疫荧光特异性试验结果
a:猪瘟病毒;b:猪繁殖与呼吸综合征病毒;c:猪伪狂犬病毒;d:猪传染性胃肠炎病毒;e:猪肠道α冠状病毒;f:猪轮状病毒;g:阳性对照;h:阴性对照
Figure 12. Specific test results of indirect immuno-fluorescenece assay
a: Classical swine fever virus; b: Porcine reproductive and respiratory syndrome virus; c: Porcine pseudorabies virus; d: Transmissible gastroenteritis virus; e: Porcine enteric α corone virus; f: Porcine rotavirus; g: Positive control; h: Negative control
图 14 间接免疫荧光试验敏感性试验结果
a:PEDV病毒液用不完全培养基DMEM按1∶10稀释;b:PEDV病毒液用不完全培养基DMEM按1∶100 000稀释;c:空白对照
Figure 14. Sensitivity test results of indirect immuno-fluorescenece assay
a:PEDV virus solution is diluted by incomplete medium DMEM at 1∶10;b:PEDV virus solution is diluted by incomplete medium DMEM at 1∶100 000;c:Blank control
表 1 间接ELISA法检测不同稀释比例病毒的抗体特异性
Table 1 Detection of antibody specificity of viruses with different dilution ratios by indirect ELISA
病毒名称1)
Virus nameD450 nm 1∶1 1∶100 1∶1 000 1∶3 000 1∶5 000 1∶10 000 1∶50 000 1∶100 000 TGEV 0.022 0.019 0.026 0.021 0.022 0.018 0.024 0.026 PRV 0.020 0.020 0.023 0.023 0.026 0.022 0.023 0.021 CSFV 0.018 0.017 0.019 0.020 0.017 0.021 0.020 0.016 PRRSV 0.018 0.020 0.023 0.021 0.017 0.020 0.022 0.018 PEDV 0.489 0.474 0.492 0.491 0.479 0.472 0.468 0.492 1)TGEV、PPRV、CSFV、PRRSV、PEDV 分别代表猪传染性胃肠炎病毒、猪伪狂犬病毒、猪瘟病毒、猪繁殖与呼吸综合征病毒、猪流行性腹泻病毒
1) TGEV, PPRV, CSFV, PRRSV and PEDV N indicate transmissible gastroenteritis virus, classical swine fever virus, porcine reproductive and respiratory syndrome virus and porcine epidemic diarrhea virus -
[1] MESQUITA J R, HAKZE-VAN D H R, ALMEIDA A, et al. Outbreak of porcine epidemic diarrhea virus in Portugal, 2015[J]. Transbound Emerg Dis, 2015, 62(6): 586-588. doi: 10.1111/tbed.12409
[2] CHEN Q, LI G, STASKO J, et al. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States[J]. J Clin Microbiol, 2014, 52(1): 234-243. doi: 10.1128/JCM.02820-13
[3] SUN J, LI Q, SHAO C, et al. Isolation and characterization of Chinese porcine epidemic diarrhea virus with novel mutations and deletions in the S gene[J]. Vet Microbiol, 2018, 221: 81-89. doi: 10.1016/j.vetmic.2018.05.021
[4] LARA-ROMERO R, GOMEZ-NUNEZ L, CERRITENO-SANCHEZ J L, et al. Molecular characterization of the spike gene of the porcine epidemic diarrhea virus in Mexico, 2013-2016[J]. Virus Genes, 2018, 54(2): 215-224. doi: 10.1007/s11262-017-1528-x
[5] ZHAO X, LI Z, ZENG X, et al. Sequence analysis of the spike gene of porcine epidemic diarrhea virus isolated from South China during 2011-2015[J]. J Vet Sci, 2017, 18(2): 237-243. doi: 10.4142/jvs.2017.18.2.237
[6] LI W, LI H, LIU Y, et al. New variants of porcine epidemic diarrhea virus, China, 2011[J]. Emerg Infect Dis, 2012, 18(8): 1350-1353. doi: 10.3201/eid1803.120002
[7] VAN DIEP N, SUEYOSHI M, NORIMINE J, et al. Molecular characterization of US-like and Asian non-S INDEL strains of porcine epidemic diarrhea virus (PEDV) that circulated in Japan during 2013-2016 and PEDVs collected from recurrent outbreaks[J]. BMC Vet Res, 2018, 14: 96. doi: 10.1186/s12917-018-1409-0.
[8] 莫炜钰, 罗均, 莫梅君, 等. 两株猪流行性腹泻野毒的确证及N基因的分析[J]. 中国兽医杂志, 2016, 52(9): 10-12. doi: 10.3969/j.issn.0529-6005.2016.09.003 [9] JUNG K, ANNAMALAI T, LU Z, et al. Comparative pathogenesis of US porcine epidemic diarrhea virus (PEDV) strain PC21A in conventional 9-day-old nursing piglets vs 26-day-old weaned pigs[J]. Vet Microbiol, 2015, 178(1/2): 31-40.
[10] LI Z L, ZHU L, MA J Y, et al. Molecular characterization and phylogenetic analysis of porcine epidemic diarrhea virus (PEDV) field strains in south China[J]. Virus Genes, 2012, 45(1): 181-185. doi: 10.1007/s11262-012-0735-8
[11] 孙东波, 冯力, 时洪艳, 等. 猪流行性腹泻病毒分子生物学研究进展[J]. 动物医学进展, 2006, 27(10): 11-14. doi: 10.3969/j.issn.1007-5038.2006.10.003 [12] KOCHERHANS R, BRIDGEN A, ACKERMANN M, et al. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence[J]. Virus Genes, 2001, 23(2): 137-144. doi: 10.1023/A:1011831902219
[13] DANIELY Y, BOROWIEC J A. Formation of a complex between nucleolin and replication protein A after cell stress prevents initiation of DNA replication[J]. J Cell Biol, 2000, 149(4): 799-810. doi: 10.1083/jcb.149.4.799
[14] 石达, 吕茂杰, 陈建飞, 等. 猪流行性腹泻病毒蛋白对Vero E6核蛋白B23.1的影响[J]. 中国兽医杂志, 2012, 48(4): 21-23. doi: 10.3969/j.issn.0529-6005.2012.04.007 [15] 石达. 猪流行性腹泻病毒核衣壳蛋白亚细胞定位信号的鉴定[D]. 哈尔滨: 中国农业科学院哈尔滨兽医研究所, 2012. [16] CHEN X, ZHANG X X, LI C, et al. Epidemiology of porcine epidemic diarrhea virus among Chinese pig populations: A meta-analysis[J]. Microb Pathog, 2019, 129: 43-49. doi: 10.1016/j.micpath.2019.01.017
[17] SUN R Q, CAI R J, CHEN Y Q, et al. Outbreak of porcine epidemic diarrhea in suckling piglets, China[J]. Emerg Infect Dis, 2012, 18(1): 161-163. doi: 10.3201/eid1801.111259
-
期刊类型引用(6)
1. 马亚娟,苏恺,林依丹,王亚文,张亚楠,袁洪兴,袁晨,宋勤叶. 盐霉素体外对猪流行性腹泻病毒的抑制效果. 畜牧兽医学报. 2024(04): 1661-1671 .  百度学术
百度学术
2. 胡泽奇,李润成,谭祖明,谢秀艳,王江平,秦乐娟,李荣,葛猛. PEDV、PoRVA和PDCoV TaqMan三重RT-qPCR检测方法的建立与初步应用. 畜牧兽医学报. 2024(05): 2267-2272 .  百度学术
百度学术
3. 路浩,丁雪燕,袁晋,魏战勇. 猪肠道冠状病毒检测方法的研究进展. 中国兽医科学. 2024(10): 1391-1398 .  百度学术
百度学术
4. 王恋春,郑辉,张慧,沙洲,房琳琳,尼博,董雅琴,魏荣,崔进,郑泽中. 猪流行性腹泻病毒实时荧光RT-RAA检测方法的建立及应用. 黑龙江畜牧兽医. 2024(20): 63-68+74 .  百度学术
百度学术
5. 宋雪莹. 猪流行性腹泻研究热点检索与分析. 福建畜牧兽医. 2023(02): 40-42 .  百度学术
百度学术
6. 李洁森,孙荣航,邝燕齐,陈路漫,刘青,郭霄峰. 猪流行性腹泻病毒S1D蛋白的优化表达及其单克隆抗体的制备. 中国畜牧兽医. 2021(12): 4641-4651 .  百度学术
百度学术
其他类型引用(18)



 下载:
下载: