Preparation of enrofloxacin nanoemulsion and evaluation of pharmacodynamic of spray administration
-
摘要:目的
以恩诺沙星为主药,研制一种可喷雾给药的纳米乳新制剂,并通过鸡全身感染疾病模型,对恩诺沙星纳米乳喷雾制剂的药效进行初步评价。
方法采用相转变法,通过单因素试验和正交试验对辅料进行筛选及处方工艺优化,采用高效液相色谱法(HPLC)对纳米乳中恩诺沙星的含量进行测定,采用纳米粒度及Zeta电位分析仪测定纳米乳粒径大小和Zeta电位,并开展人工诱发鸡白痢沙门氏菌病和禽大肠埃希菌病感染模型的药效学试验,评估恩诺沙星纳米乳喷雾给药对常见细菌性疾病的防治效果。
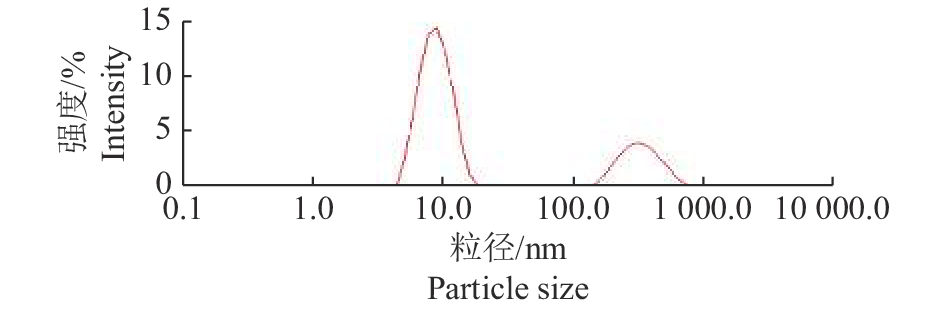
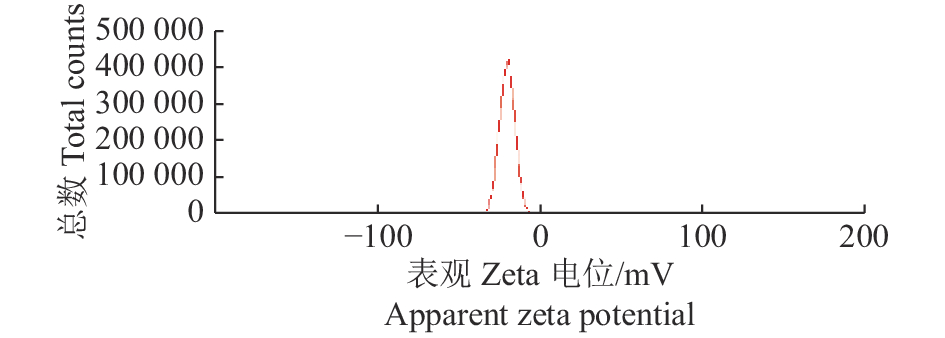
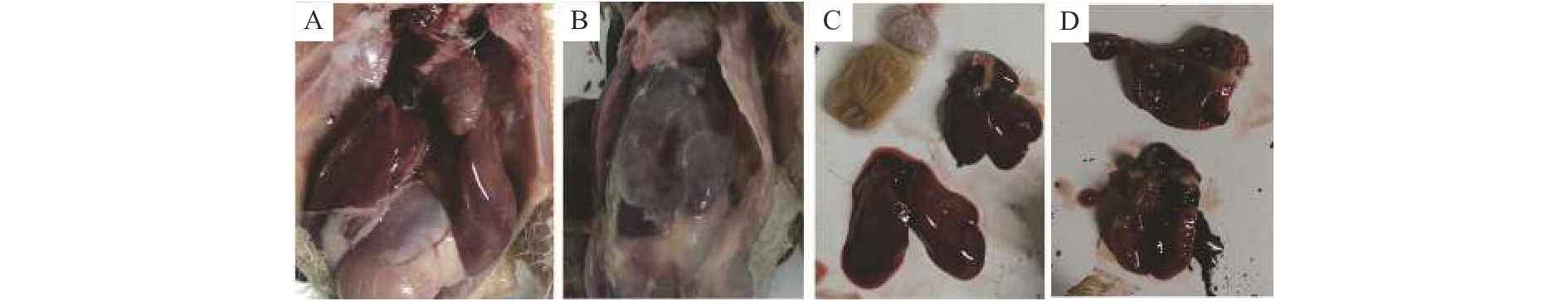
结果本试验所制备的纳米乳为水包油型,呈淡黄色,澄清透明,乳滴分布均匀,平均粒径为(10.79±1.5) nm,多分散系数(PDI)为0.446,Zeta电位为−21.63 mV。药效学评估显示:高、中、低剂量预防给药组的存活率分别为90.0%、83.3%、73.3%,均显著高于感染对照组(26.7%)(P<0.01)。
结论本研究制备的复方恩诺沙星纳米乳性质稳定,超声雾化喷雾给药对沙门氏菌和大肠埃希菌引起的鸡全身感染有良好的防治效果。
Abstract:ObjectiveTo develop a new formulation of enrofloxacin nanoemulsion for spray administration, and evaluate its disease control effect using chicken model of systemic infection.
MethodThe phase transition method was used, and single factor and orthogonal tests were performed to screen formulations and optimize the prescription. High-performance liquid chromatography (HPLC) was used to determine enrofloxacin content in nanoemulsion. Nanoparticle and zeta potential analyzer was used to measure the particle size and zeta potential of nanoemulsion. Pharmacodynamic experiments were performed using chicken models infected by Salmonella pullorum and Escherichia coli, and the control effects of spray adminstration of compound enrofloxacin nanoemulsion on bacterial diseases were evaluated.
ResultThe nanoemulsion prepared in this experiment was an oil-in-water type, light yellow, clear and transparent, and had uniform droplet distribution. The average particle size was (10.79 ± 1.5) nm, the polydispersity index (PDI) was 0.446 and the zeta potential was −21.63 mV. The pharmacodynamic evaluation showed that the survival rates of preventive administration groups with high, medium and low doses were 90.0%, 83.3% and 73.3% respectively, which were significantly higher than that of the infection control group (26.7%) ( P<0.01).
ConclusionThe compound enrofloxacin nanoemulsion prepared in this study has stable properties. The ultrasonic spray administration has good control effects on chicken diseases due to systemic infection of Salmonella pullorum and Escherichia coli.
-
Keywords:
- enrofloxacin /
- nanoemulsion /
- spray administration /
- poultry /
- Salmonella pullorum /
- Escherichia coli
-
-
表 1 动物分组及试验处理
Table 1 Animal grouping and experimental treatment
处理
Treatment组别
Group鸡只数量
Chicken number体质量/g
Body
weightρ(药物)/(mg·L−1)
Drug concentration每日给药次数
Times of daily administration不感染,不给药 No infection, no medication 健康对照组 Healthy control group 30 242.1±21.2 只感染,不给药 Infection only, no medication 感染对照组 Infection control group 30 232.5±26.8 喷雾给药3 d 后攻毒感染,再喷雾给药5 d
Infection after spray administration for 3 days, then spray administration for 5 days低剂量预防给药组
Low dose preventive administration group30 233.7±27.3 112 2 中剂量预防给药组
Medium dose preventive administration group30 234.3±25.6 225 2 高剂量预防给药组
High dose preventive administration group30 233.8±26.5 450 2 先感染,再喷雾给药5 d
Infection first, then spray administration for 5 days治疗给药组
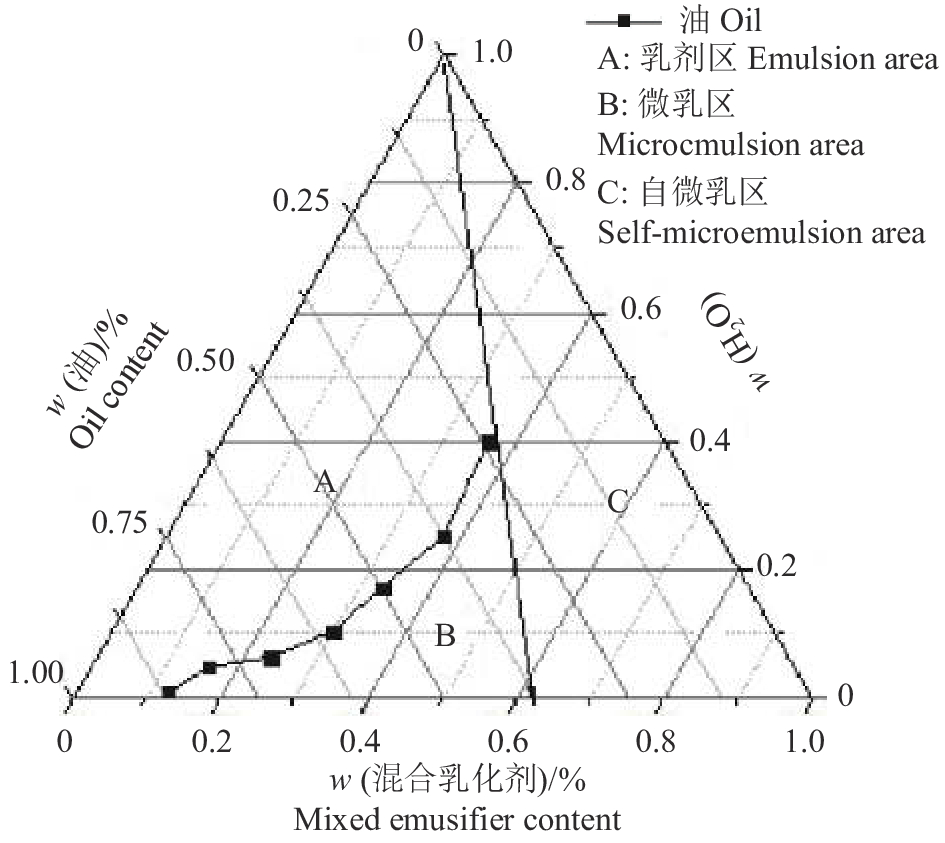
Treatment administration group30 235.3±23.6 225 2 表 2 乳化剂与助乳化剂体积比(Km)为3∶2时滴定过程数据记录
Table 2 Data of titration with water when the volume fraction of emusifier to coemusifier(Km) was 3∶2
V(吐温−80)/mL
Tween-80 volumeV(无水乙醇)/mL
Anhydrous ethanol volumeV(桉油)/mL
Eucalyptus oil volumeV滴定(H2O)/mL
Volume of water for titration混合乳化剂∶油相(V/V)
Mixed emulsifier∶Oil phase6 4.00 3.33 无限稀释 Infinite dilution 3∶1 6 4.00 5.00 无限稀释 Infinite dilution 2∶1 6 4.00 6.66 11.00 3∶2 6 4.00 10.00 6.70 1∶1 6 4.00 15.00 5.20 2∶3 6 4.00 20.00 3.50 1∶2 6 4.00 30.00 2.70 1∶3 1 0.67 8.35 0.50 1∶5 1 0.67 11.69 0.10 1∶7 表 3 乳化剂与助乳化剂体积比(Km)为3∶2时pH对纳米乳的影响
Table 3 The effects of pH on nanoemulsion when the volume fraction of emusifier to coemusifier(Km) was 3∶2
pH 颜色及澄明度
Color and clarity分层
Layering药物析出
Drug precipitationw(药物)/%
Drug content9.3 淡黄色 Pale yellow 无 No 有 Yes 9.5 淡黄色,澄明
Pale yellow, clear无 No 无 No 4.90 9.7 淡黄色,澄明
Pale yellow, clear无 No 无 No 4.97 10.0 淡黄色,澄明
Pale yellow, clear无 No 无 No 4.91 10.2 淡黄色,澄明
Pale yellow, clear无 No 无 No 4.91 10.5 淡黄色,澄明
Pale yellow, clear无 No 无 No 4.91 10.7 乳白色,不透明
Milky white, opaque有 Yes 无 No 表 4 恩诺沙星纳米乳正交试验处方筛选结果
Table 4 Screened formula of enrofloxacin nanoemulsion by orthogonal test
抗氧化剂
Antioxidantρ(抗氧化剂)/
(g·L−1)
Antioxidant content混合乳化剂∶
油相(V/V)
Mixed emulsifier∶
Oil phase药物析出
Drug
precipitationw(药物)/%
Drug
content亚硫酸氢钠
Sodium
bisulfite2 5∶2 无 No 5.24 2 3∶1 无 No 5.04 2 4∶1 无 No 5.18 4 5∶2 无 No 4.87 4 3∶1 无 No 4.82 4 4∶1 无 No 5.29 5 5∶2 微量 Trace 5 3∶1 微量 Trace 5 4∶1 微量 Trace 焦亚硫酸钠
Sodium
pyrosulfite2 5∶2 无 No 5.12 2 3∶1 无 No 4.94 2 4∶1 无 No 4.75 4 5∶2 无 No 4.96 4 3∶1 无 No 5.74 4 4∶1 无 No 5.00 5 5∶2 微量 Trace 5 3∶1 微量 Trace 5 4∶1 微量 Trace 表 5 各试验组的预防及治疗效果
Table 5 Preventive and therapeutic effects of each trial
组别1)
Group感染 Infected 病死 Death 保护率2)/%
Protection rate治愈率3)/%
Cure rate14 d后增质量/g
Gain weight after 14 days数量 Number 比例/% Rate 数量 Number 比例/% Rate 1 0 0 0 0 143.2±28.8 2 30 100 22 73.3 26.7a 97.5±21.2 3 14 46.7 8 26.7 53.3(16/30) 35.7(5/14) 112.7±23.3 4 8 26.7 5 16.7 73.3(22/30) 37.5(3/8) 121.8±26.9 5 5 16.7 3 10.0 83.3(25/30) 40.0(2/5) 136.7±30.9 6 29 96.7 15 50.0 51.7b(15/29) 37.9(11/29) 117.9±17.1 1) 1~6分别为健康对照组、感染对照组、低剂量预防给药组、中剂量预防给药组、高剂量预防给药组和治疗给药组;2)括号中的数值为治愈数量与感染数量比值;“a”表示感染组的自愈率;“b”表示治疗组的有效率
1) 1−6 are healthy control group, infection control group, low dose preventive administration group, medium dose preventive administration group, high dose preventive administration group and treatment administration group; 2) The value in brackets is the ratio of the number of cured to the number of infected; “a” indicates the self-healing rate of the infection group; “b” indicates the effective rate of the treatment group -
[1] SAIF Y M, 主编. 禽病学: 12版[M]. 苏敬良, 高福, 索勋, 译. 北京: 中国农业出版社, 2012: 811-870. [2] 崔福德, 主编. 药剂学: 6版[M]. 北京: 人民卫生出版社, 2010: 201-213. [3] CALDERON-NIEVA D, GOONEWARDENE K B, GOMIS S, et al. Veterinary vaccine nanotechnology: Pulmonary and nasal delivery in livestock animals[J]. Drug Deliv Transl Re, 2017, 7(4): 558-570. doi: 10.1007/s13346-017-0400-9
[4] VARGA M. Therapeutics[M]//Textbook of Rabbit Medicine: 2nd ed. Oxford: Butterworth-Heinemann, 2014: 137-177.
[5] PERRY S M, MITCHELL M A. Antibiotic therapy[M]//Mader's reptile and amphibian medicine and surgery: 2nd Edition. Philadelphia: Saunders, 2019: 1139-1154.
[6] 陈杖榴. 兽医药理学: 3版[M]. 北京: 中国农业出版社, 2004. [7] MARTINEZ M, MCDERMOTT P, WALKER R. Pharmacology of the fluoroquinolones: A perspective for the use in domestic animals[J]. The Veterinary Journal, 2006, 172(1): 10-28. doi: 10.1016/j.tvjl.2005.07.010
[8] 方炳虎, 曾振灵, 冯淇辉, 等. 恩诺沙星对鸡大肠杆菌病及葡萄球菌病的药效研究[J]. 中国兽医学报, 1997(2): 54-57. [9] GAUTIER-BOUCHARDON A V, REINHARDT A K, KOBISCH M, et al. In vitro development of resistance to enrofloxacin, erythromycin, tylosin, tiamulin and oxytetracycline in Mycoplasma gallisepticum, Mycoplasma iowae and Mycoplasma synoviae[J]. Veterinary Microbiology, 2002, 88(1): 47-58. doi: 10.1016/S0378-1135(02)00087-1
[10] SCHRODER J. Enrofloxacin: A new antimicrobial agent[J]. Journal of the South African Veterinary Association, 1989, 60(2): 122-124.
[11] FLECKENSTEIN E, UPHOFF C C, DREXLER H G. Effective treatment of Mycoplasma contamination in cell lines with enrofloxacin (Baytril)[J]. Leukemia, 1994, 8(8): 1424-1434.
[12] BAUDITZ R. Results of clinical studies with Bytril in poultry[J]. Veterinary Medical Review, 1987, 2: 130-136.
[13] SUMANO L H, OCAMPO C L, BRUMBAUGH G W, et al. Effectiveness of two fluoroquinolones for the treatment of chronic respiratory disease outbreak in broilers[J]. British Poultry Science, 1998, 39(1): 42-46. doi: 10.1080/00071669889376
[14] 王波臻, 欧阳五庆, 纪俊明, 等. 复方恩诺沙星纳米乳的制备及体外透皮性能考察[J]. 畜牧与兽医, 2016, 48(3): 77-83. [15] 毛庆祥, 常文军, 蔡全才. 桉叶油透皮吸收促进剂研究进展[J]. 药学实践杂志, 2003(4): 205-209. doi: 10.3969/j.issn.1006-0111.2003.04.005 [16] DHAKAD A K, PANDEY V V, BEG S, et al. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review[J]. Journal of the Science of Food and Agriculture, 2018, 98(3): 833-848. doi: 10.1002/jsfa.8600
[17] 朱向波. 鸡白痢沙门氏菌病诊断与防治[J]. 中国畜禽种业, 2015, 11(5): 149-150. doi: 10.3969/j.issn.1673-4556.2015.05.112 [18] 郑志新. 张家口地区鸡白痢沙门氏菌的分离鉴定及药敏试验[J]. 河北北方学院学报(自然科学版), 2015, 31(3): 36-38. [19] 安辉, 张大华, 陈小玲, 等. 鸡白痢沙门氏菌的分离与鉴定[J]. 安徽农业科学, 2011, 39(32): 19888-19890. doi: 10.3969/j.issn.0517-6611.2011.32.091 [20] 刘吉山, 沈志强, 王燕, 等. 我国部分地区禽致病性大肠杆菌的分离鉴定与药敏试验[J]. 中国家禽, 2005(7): 14-16. doi: 10.3969/j.issn.1004-6364.2005.07.004 [21] 路卫星. 鸡大肠杆菌病的诊断与治疗[J]. 中国兽医杂志, 2015, 51(8): 43-44. doi: 10.3969/j.issn.0529-6005.2015.08.015 [22] 陈洪科. 禽病诊断和防治: 1版[M]. 北京: 中国农业科技出版社, 1996. [23] 刘慕欣, 付领新. 喷雾给药治疗禽类呼吸道疾病的效果观察[J]. 当代畜牧, 2014(8): 76-77. [24] DAS A, AHMED A B. Natural permeation enhancer for transdermal drug delivery system and permeation evaluation: A review[J]. Asian Journal of Pharmaceutical and Clinical Research, 2017, 10(9): 5. doi: 10.22159/ajpcr.2017.v10i9.19389
[25] 陈宇. 桉叶挥发油制剂开发及其抗慢性支气管炎的药效研究[D]. 天津: 天津大学, 2008. [26] SADLON A E, LAMSON D W. Immune-modifying and antimicrobial effects of Eucalyptus oil and simple inhalation devices[J]. Alternative Medicine Review: A Journal of Clinical Therapeutic, 2010, 15(1): 33.
[27] 欧阳五庆, 杨增岐, 李雅. 恩诺沙星对人工诱发的鸡大肠杆菌病的疗效[J]. 西北农业大学学报, 1998: 76-79.



 下载:
下载: