Preparation and in vitro release characteristics of florfenicol sustained-release granules
-
摘要:目的
延长药物有效血药浓度的维持时间,产生持续稳定的抑菌作用,避免多剂量连续使用氟苯尼考产生胚胎毒性和免疫抑制。
方法以硬化油、聚乙二醇、甘油酯为辅料,采用离心喷雾干燥制粒技术法制备氟苯尼考缓释颗粒,高效液相色谱法(HPLC)测定氟苯尼考含量。以药物在不同释放介质(pH 1.2盐酸缓冲液、pH 4.3醋酸缓冲液和pH 6.8磷酸缓冲液)中的释放特性为指标,对氟苯尼考粉和自制的氟苯尼考缓释颗粒进行溶出试验,考察体外释放特性。
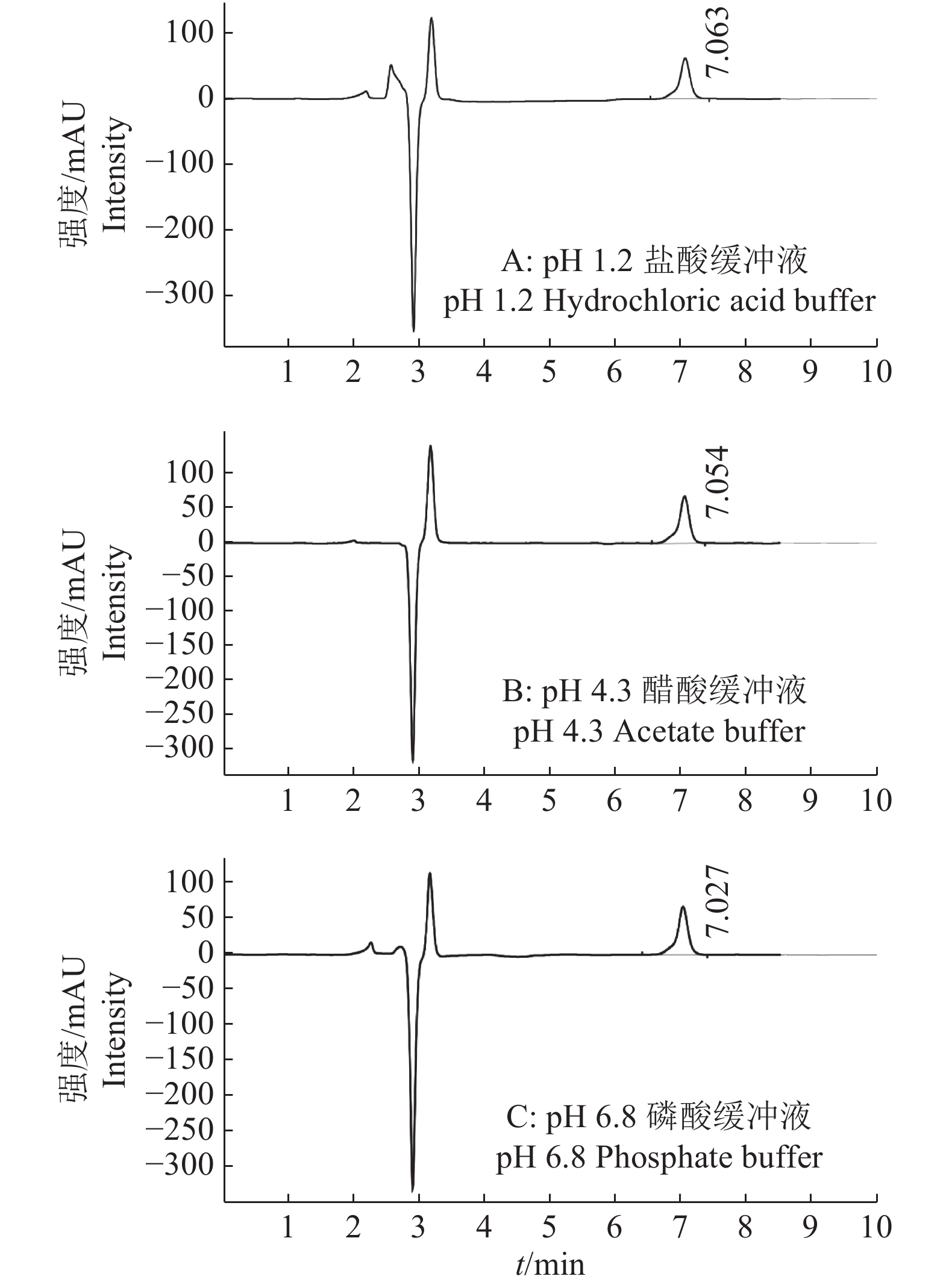
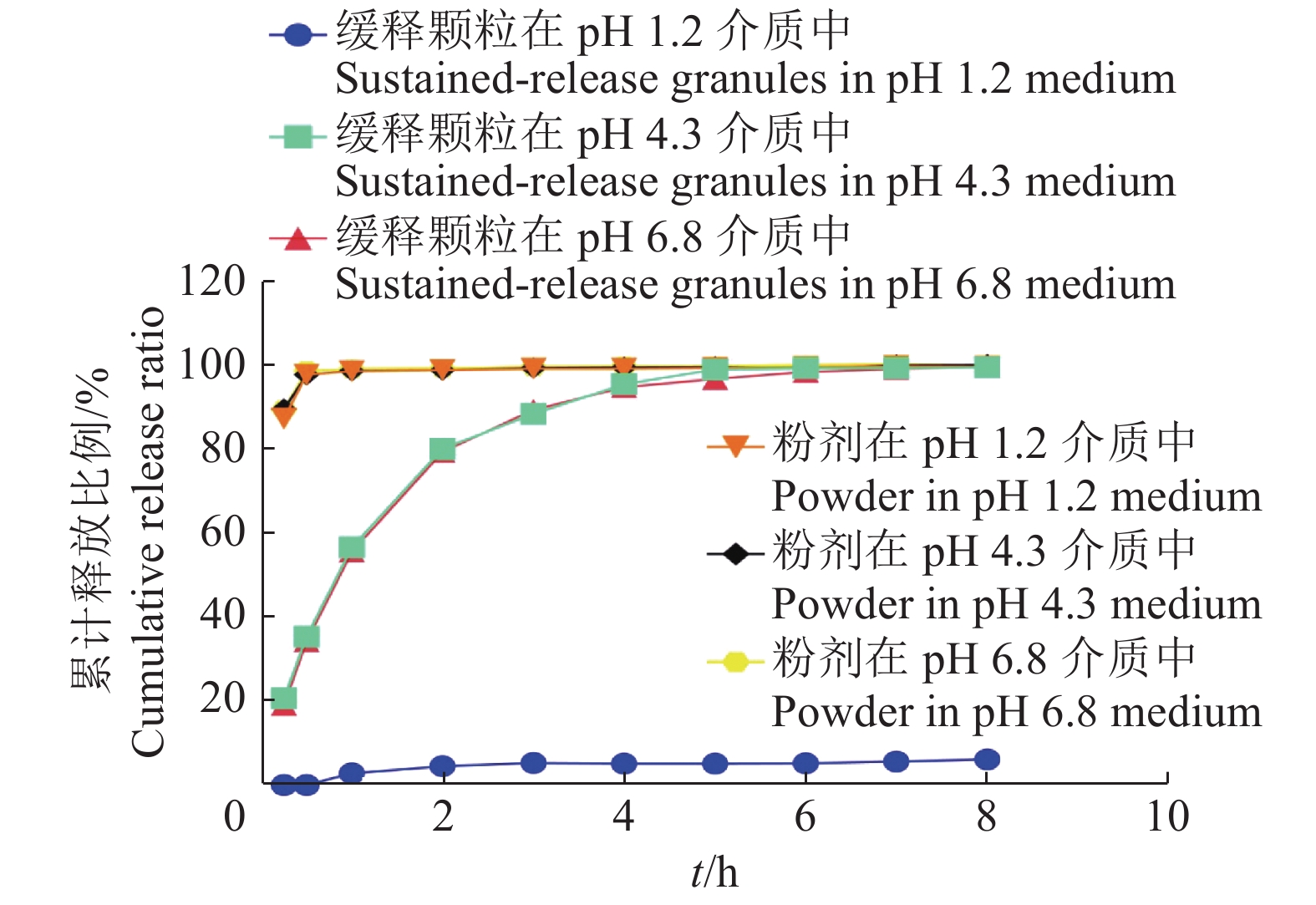
结果4个批次制备的氟苯尼考缓释颗粒中氟苯尼考实际含量在标示含量中的占比分别为99.19%、100.01%、97.45%和100.72%,相对标准偏差(RSD)分别为0.82%、0.86%、0.77%和0.24%。氟苯尼考粉在0.25 h内完全释放,自制的氟苯尼考缓释颗粒在模拟胃液环境(pH 1.2盐酸缓冲液)基本不释放,在模拟肠液环境(pH 4.3醋酸缓冲液和pH 6.8磷酸缓冲液)缓慢释放,5 h内释放量达95%以上。
结论自制的氟苯尼考缓释颗粒中药物分布较均匀,符合中国兽药典要求。相比于氟苯尼考粉,氟苯尼考缓释颗粒表现出良好的耐酸性体外缓释性,可为后续的临床试验研究和临床使用药物提供参考依据。
Abstract:ObjectiveTo prolong the maintenance time of the effective blood concentration of the drug, generate a sustainable and stable bacteriostatic action, and avoid embryotoxicity and immunosuppression in multi-dose continuous use of florfenicol.
MethodThe test used hardened oil, polyethylene glycol and glyceride as auxiliary materials. The florfenicol sustained-release granules were prepared by centrifugal spray drying granulation method, and the content of florfenicol was determined by high performance liquid chromatography (HPLC). Using release characteristics of the drug in different media (pH 1.2 hydrochloric acid buffer, pH 4.3 acetate buffer and pH 6.8 phosphate buffer) as indicators, the dissolution tests of florfenicol powder and self-made florfenicol sustained-release granules were carried out to investigate their in vitro release performances.
ResultThe percentages of florfenicol actual contents in indicated contents of florfenicol sustained-release granules prepared in four batches were 99.19%, 100.01%, 97.45% and 100.72%, and the relative standard deviations (RSD) were 0.82%, 0.86%, 0.77% and 0.24% respectively. The florfenicol powder was completely released within 0.25 h. The self-made florfenicol sustained-release granules were substantially not released in simulated gastric fluid environment (pH 1.2 hydrochloric acid buffer), and released slowly under simulated intestinal fluid environment (pH 4.3 acetate buffer and pH 6.8 phosphate buffer) with the release amount of over 95% within 5 h.
ConclusionThe drug in self-made florfenicol sustained-release granules is homogeneous distributed, and the granules conform to China veterinary pharmacopoeia. Compared with florfenicol powder, florfenicol sustained-release granules show good acid resistance and in vitro sustained-release performance, which provides a reference for subsequent clinical trial research and clinical use of drugs.
-
鱼类通过消化系统完成对食物的摄取和营养物质的消化吸收.鱼类的早期发育阶段是消化系统不断发育和完善的时期,随着仔鱼食性的转变,消化系统会发生相应的变化,以满足仔鱼生长发育所必需的营养需求.所以在鱼类的早期发育时期消化系统的发育状况会影响到鱼苗的成活率以及鱼苗的发育情况.因此,很有必要研究鱼类早期发育阶段消化系统的形成和发育过程,以便更合理地去配制饵料,提供仔鱼生长的最适饵料,使鱼苗能更好地生长发育,达到更高的成活率.Mai等[1]研究了对大黄鱼Pseudosciaena crocea仔、稚鱼的消化系统,描述了0~40 d鱼消化道的形态组织学特征.Ostaszewska等[2]观察了尖吻鲟Acipenser oxyrinchus孵化后6~49 d消化道的形态组织变化.Saelee等[3]报道了连鳍胡鲇Clarias nieuhofii孵化后0~46 d消化系统发育的组织学和组织化学特性.区又君等[4]研究了0~31 d卵形鲳鲹Trachinotus ovatus消化器官的发育过程及组织结构特征.
条石鲷Oplegnathus fasciatus在分类学上隶属于鲈形目Perciformes石鲷科Oplegnathidae石鲷属,俗称日本鹦鹉鱼,属近海暖温、喜岩礁性鱼类,分布于中国、日本,我国产于黄海、东海、台湾海峡等地[5-6],该鱼色泽鲜艳、条纹美丽,是一种具有较高经济价值和观赏价值的名贵海产鱼类.国内外有关条石鲷生物学的研究已有不少[7-9],胡玲玲等[10]采用解剖和光镜技术对养殖条石鲷消化道的形态学和组织学进行了详细的观察和研究,并探讨了其消化道的组织学和解剖学特征与其杂食性的适应.本文对条石鲷仔、稚、幼鱼消化系统的组织学进行研究,以期为该物种的发育生物学研究和种苗生产提供技术支撑.
1. 材料与方法
1.1 材料
试验用鱼取自南海水产研究所饶平试验基地.育苗水温为24~27 ℃,盐度29~30,pH 7.4~8.2.从0日龄(孵出第1天)仔鱼开始取样观察,直到35日龄,所取鱼苗均健康无病,活动状态正常.样品用波恩氏液进行固定,置于体积分数为70%的乙醇溶液中保存.
1.2 方法
按日龄由小到大取样切片.将已经固定好的标本用自来水冲洗2 h,利用TP1020自动脱水机脱水,透明和浸蜡,用组织包埋机石蜡包埋,Leica轮转式切片机进行纵向和横向连续切片,切片的厚度均为5 μm,贴片,烤片.将制作好的石蜡切片放入Autostainer XL自动染色机内进行H-E染色,中性树胶封片,用卡尔·蔡司光学显微镜进行观察,并摄影.利用目镜测微尺测量各组织长度.
2. 结果
2.1 前期仔鱼(0~3日龄)
初孵仔鱼(0日龄仔鱼),消化系统尚未分化,仅有一段紧贴卵黄囊与腹腔壁的尚未分化的肠管,无黏膜层和黏膜下层、肌肉层的分化.细胞的界线不清晰,由立方形细胞构成,细胞核大,几乎占据整个细胞.口咽腔和肛门尚未形成.此时仔鱼的卵黄囊体积很大,H-E染色呈淡红色,仔鱼以卵黄物质为营养来源,处于内源性营养阶段(图 1a).
![]() 图 1 条石鲷仔、稚、幼鱼消化系统组织结构观察(H-E染色)BC:口咽腔;DT:消化道;TB:味蕾;T:舌;PST:雏形胃;ES:食道;GC:杯状细胞;BB:纹状缘;MC:黏膜层;MS:肌肉层;ST:胃;AN:肛门;P:胰脏;L:肝脏;V:肝空泡;VS:静脉窦;SE:浆膜层;IL:胰岛;GAC:鳃弓软骨;BA:鳃弓;FG:假鳃;UJ:上颌;LJ:下颌;AI:前肠;UP:上咽.
图 1 条石鲷仔、稚、幼鱼消化系统组织结构观察(H-E染色)BC:口咽腔;DT:消化道;TB:味蕾;T:舌;PST:雏形胃;ES:食道;GC:杯状细胞;BB:纹状缘;MC:黏膜层;MS:肌肉层;ST:胃;AN:肛门;P:胰脏;L:肝脏;V:肝空泡;VS:静脉窦;SE:浆膜层;IL:胰岛;GAC:鳃弓软骨;BA:鳃弓;FG:假鳃;UJ:上颌;LJ:下颌;AI:前肠;UP:上咽.
a:0 d仔鱼纵切;b:1 d仔鱼纵切;c:2 d仔鱼肝脏原基纵切;d:2 d仔鱼雏形胃纵切;e:2 d仔鱼消化管纵切;f:4 d仔鱼口咽腔纵切;g:5 d仔鱼肝脏纵切;h:8 d仔鱼消化道纵切;i:8 d仔鱼鳃横切;j:10 d咽腔纵切;k:12 d仔鱼整体横切;l:15 d肝脏纵切;m:15 d食道横切;n:15 d胰脏纵切;o:15 d舌纵切;p:15 d胃纵切;q:17 d食道横切;r:17 d口咽腔纵切;s:18 d胃腺纵切;t:18 d肝脏纵切;u:25 d口咽腔纵切;v:35 d鳃纵切;w:35 d食道横切.Figure 1. Histological observation on the digestive system of larvae, juvenile and young Oplegnathus fasciatus(H-E staining)1日龄仔鱼,肠管较初孵仔鱼变粗变长,肠前后部开始出现空腔,细胞体积变大,数目增多,细胞界限不明显,排列较不规则,细胞核仍很大.肛门基本形成,但仍未与外界相通.卵黄囊体积减小(图 1b).
2日龄仔鱼,口裂形成,但还没有开启能力;在口腔形成处出现少量的染成蓝色的软骨细胞,下颌骨开始骨化;食道仍未贯通,细胞排列杂乱,外被浆膜.食道后部的消化道黏膜下层和肌肉层没有形成;在卵黄囊背部出现一染色较深的细胞团,为肝脏原基;胃原基形成空腔,黏膜层出现少量细小的凹陷,腔上皮为单层立方上皮,细胞排列整齐,细胞界限较清晰,细胞核较大,圆形;肠管继续增长,贯穿整个体腔前后,肠腔从两端向中间扩展,肠道未与外界相通,肠黏膜上皮细胞呈柱状,在细胞顶端已形成排列整齐的微绒毛;肾脏开始形成;肝脏原基正在分化形成,肝细胞界限不清晰(图 1c,1d,1e).
2.2 开口期仔鱼(4~8日龄)
4日龄仔鱼,卵黄囊消失,已开口,消化道与外界相通,形成口咽腔(图 1f)、食管、胃、肠和肛门,开始摄食.食道和胃的连接处出现缢痕.胃部开始膨大,肠道增粗,变弯;胃黏膜形成5~6个低褶,黏膜外形成一层结缔组织.肝细胞呈不规则的多边形.
5日龄仔鱼,口咽腔继续扩大;肠道仍无明显的前、中、后肠的分化,肠壁未见肌肉层,肠上皮细胞分裂加快;肝细胞增多,排列较松散(图 1g).
8日龄仔鱼,口咽腔和食道的黏液细胞迅速增多,肌肉层也迅速增厚;出现短的鳃丝,形成1列假鳃;食管形成7个皱襞,肌层增厚;胃的两端均出现紧缢,使胃与食道、肠的分界明显,胃黏膜褶增至7~9个,黏膜下层含有较多的淋巴细胞;肝脏体积增加,肝细胞增多,排列较致密;肾小管细胞增多,体积增大(图 1h,1i).
2.3 后期仔鱼(10~25日龄)
10日龄仔鱼,下颌宽12.5 μm,出现味蕾;咽部高162.5 μm,出现味蕾;肠黏膜层细胞质中出现大量的嗜伊红颗粒和空泡,肝脏体积显著增大,横切宽为172.5 μm,食道表层上皮细胞之间出现大量的杯状细胞.假鳃长度增到120 μm(图 1j).
12~15日龄仔鱼,口咽腔黏膜层增厚,舌上出现大量味蕾,咽部味蕾增多,鳃丝迅速发育,最长达到270 μm,鳃弓上出现很多味蕾;食道肌层增厚,食道长为135 μm;形成胃腺和幽门盲囊,胃黏膜层增厚,胃小凹增多;肠绒毛明显增长和密集;肝胰脏基本发育成形,肝内的空泡增多(图 1k,1l,1m,1n,1o,1p).
17~25日龄,此时期条石鲷仔鱼的支鳍骨开始形成,各鳍开始进入快速发育时期,25日龄为仔鱼到稚鱼的转变期.胃腺增多,胃肌层进一步增厚,胃和肠壁形成不连续的纵行平滑肌肉层(图 1q,1r,1s,1t,1u).
2.4 稚鱼期(25~35日龄)
进入稚鱼阶段,在舌上出现杯状细胞和味蕾,上下颌的表皮层都含有大量的杯状细胞;胃分化为幽门部,盲囊部和贲门部,胃的柱状上皮顶端有不规则的突起;肠肌层厚度约为62.5 μm.消化系统的各部分结构和功能基本发育完善,消化能力明显增强(图 1v,1w).
3. 讨论
3.1 条石鲷消化系统的早期发育阶段划分
条石鲷消化系统的早期发育与其他的鱼类相似,初孵仔鱼消化系统尚未分化,到开口摄食时,为了适应外源性营养方式,消化系统迅速分化而向仔鱼后期发育,具备基本的结构[11].可能是仔鱼对卵黄物质的吸收只需要基本的系统结构[12].根据形态结构观察以及组织切片的观察,把条石鲷消化系统早期发育分成3个阶段.
第1阶段为孵化后0~3日龄,此时期,仔鱼尚未开口,消化道不与外界相通,仔鱼以卵黄物质为营养,处于内源性营养阶段,消化系统开始发育但尚未分化.要提高这一阶段仔鱼的成活率,除了提供适宜的环境条件,还要注意产前雌性亲鱼的卵的质量,加强亲鱼的培育.雌性产卵群体的饵料质量与仔鱼的饵料在影响仔鱼成活率方面起着同等重要的作用[13].
第2阶段,4~18日龄,卵黄囊消耗完,仔鱼开始摄食,进入外源性营养阶段.消化系统初步发育成型,具备基本的结构,消化系统分化形成口咽腔、食管、胃、肠和肛门,形成肝胰脏.具一定的摄食、储存、消化和吸收的功能.该阶段鱼苗要经历从内源性营养到外源性营养的转变.这个阶段也是鱼苗死亡率较高的时期,由于消化系统发育还不完善,因此要提供适合鱼苗口径以及消化系统特点的饵料.
第3阶段,19~35日龄,胃腺和幽门盲囊已经形成,消化系统已具备成鱼的结构,胃腺的完善促进了食物的消化,这一阶段鱼苗的鳍和鳞片也相继发育完成,这都保证了鱼苗能够快速地生长发育.
3.2 嗜伊红颗粒、空泡和杯状细胞在消化吸收过程中的作用
肠道上皮细胞顶端出现的嗜伊红颗粒和空泡,是细胞消化的一种形式[14].由于在仔鱼期,鱼类的消化系统发育尚不完全,所以仔鱼通过这种途径来实现对营养物质的吸收,尽管这种蛋白质的细胞内消化速度很慢,但对仔鱼摄取营养起到了重要的作用.
食道中的杯状细胞能够分泌黏液,一方面可以起到润滑食道的作用,另一方面可以起到胃前消化的作用.在食道上皮层中含有大量的杯状细胞,而胃的上皮层中不含有杯状细胞.
本研究中,条石鲷10日龄仔鱼,在咽部出现高162.5 μm的味蕾,肠黏膜层细胞质中出现大量的嗜伊红颗粒和空泡,表明此阶段仔鱼的消化道上皮细胞已具有胞饮作用和细胞内消化作用.有文献推测,前肠和中肠的空泡为被吸收的脂肪滴,而在后肠的内容物则是通过胞饮作用吸收的蛋白质.在硬骨鱼类的仔鱼期阶段,由于消化酶系统发育尚未完善,胞饮吸收可能成为鱼体消化蛋白质的一条替代途径[15].在12日龄仔鱼的肝中可见有许多空泡,表明条石鲷仔鱼将从食物中吸收的营养物质贮藏于肝脏中.有研究人员通过PAS染色结果表明,这些空泡为储存在肝脏中的糖元[16].10日龄仔鱼,食道表层上皮细胞之间出现大量的杯状细胞,孵化后25 d在舌上可见到杯状细胞和味蕾,上下颌的表皮层都含有大量的杯状细胞,在胃的黏膜层中未发现杯状细胞.杯状细胞分泌黏液可能具有两方面的作用,一是起润滑作用便于食物顺利通过消化道,另一方面则可能起着胃前消化的作用.
3.3 条石鲷消化道胚后发育组织学特征与其功能的关系
条石鲷消化道的形态组织结构及其功能随着鱼体发育不断完善.孵化后2日龄的仔鱼处于内源性营养阶段,此时尚未开口,其消化道仅为一条实心管道,但已足以满足仔鱼的生理需求.这与高露姣等[17]对银鲳Pampus argenteus的研究结果相一致,而与林强等[18]对大海马Hippocampus kuda的研究结果不同,大海马在仔鱼期已具备可以独立摄食、比较完善的摄食和消化器官,仔鱼的摄食方式未经过内源性营养阶段,直接进入内源与外源混合的营养阶段.4~8日龄条石鲷的仔鱼已经开口,肛门和外界相通,消化道各器官已基本分化,随着发育进程,食道黏膜的褶皱数增多、胃容扩张、肠壁膨大等,这与马爱军等[19]对黑鲷Sparus macrocephalus以及陈晓武等[20]对牙鲆Paralichthys olivaceus的研究观察结果大体相似.表明此阶段的仔鱼已经开口向外界摄食,消化道组织结构上应有一定的发育变化才能满足其生存需求.食道黏膜层具有褶皱,可扩大食道的容积,以利于食物顺利通过食道;胃部扩张使仔鱼获得贮存更多食物的空间;幽门盲囊的分化和肠道膨大都有利于食物的充分消化和吸收.
稚鱼期条石鲷消化道已经表现出该种固有的特性,具有较强的消化功能.食道管肌层增厚,黏膜褶皱加深,黏膜上皮中有杯状细胞,这些结构特征使稚鱼能顺利地吞食较大食物并能成功转移到胃部;胃分化为幽门胃,胃体部和贲门胃,有利于鱼将所摄入的坚硬或难以消化食物在酸性环境中泡软消化,幽门部肌肉层增厚有助于通过收缩肌肉挤压磨碎食物,并将其顺利送入至容积较小的肠道;幽门盲囊与肠道分化相似,黏膜褶皱和杯状细胞数量增多能促使稚鱼对食物的消化吸收更为完全.这与吴金英等[21]对斜带石斑鱼Epinephelus coioide和徐晓津等[22]对大黄鱼的研究结果相似.杯状细胞分泌的黏液一般为中性黏液,具有润滑食物、保护肠道的功用,还可与消化酶协同作用帮助消化[23].黏膜上皮是单层柱状细胞,具有明显的纹状缘,能促进蛋白质、脂肪和糖类等消化分解成为可溶性小分子物质而被肠壁吸收[24].
-
表 1 添加制备氟苯尼考缓释颗粒空白辅料的药物回收率
Table 1 Recovery rates of blank excipients for preparation of florfenicol sustained-release granules
n=3 ρ/(μg/mL)
释放介质
Release medium回收率/% Recovery rate RSD 1 2 3 ${\bar{\textit{X} } } \pm {\rm{SD} }$ 40 pH 1.2 盐酸缓冲液
pH 1.2 Hydrochloric acid buffer98.33 98.50 98.63 98.49±0.12 0.13 pH 4.3 醋酸缓冲液
pH 4.3 Acetate buffer98.47 100.07 100.47 99.67±0.86 0.86 pH 6.8 磷酸缓冲液
pH 6.8 Phosphate buffer100.00 100.91 99.28 100.06±0.67 0.67 50 pH 1.2 盐酸缓冲液
pH 1.2 Hydrochloric acid buffer100.74 99.46 100.98 100.39±0.67 0.67 pH 4.3 醋酸缓冲液
pH 4.3 Acetate buffer100.33 101.13 100.28 100.58±0.39 0.39 pH 6.8 磷酸缓冲液
pH 6.8 Phosphate buffer99.35 100.03 100.22 99.87±0.37 0.37 60 pH 1.2 盐酸缓冲液
pH 1.2 Hydrochloric acid buffer98.93 98.29 98.70 98.64±0.26 0.27 pH 4.3 醋酸缓冲液
pH 4.3 Acetate buffer100.84 100.13 100.93 100.63±0.36 0.35 pH 6.8 磷酸缓冲液
pH 6.8 Phosphate buffer98.82 100.40 101.03 100.08±0.93 0.93 表 2 添加制备氟苯尼考缓释颗粒空白辅料的药物精密度
Table 2 Accuracy of blank excipients for preparation of florfenicol sustained-release granules
n=6 释放介质
Release medium氟苯尼考含量/% Florfenicol content RSD/% 1 2 3 4 5 6 ${\bar{\textit{X} } } \pm {\rm{SD} }$ pH 1.2 盐酸缓冲液
pH 1.2 Hydrochloric acid buffer100.60 101.02 100.95 99.32 98.87 99.51 100.05±0.84 0.84 pH 4.3 醋酸缓冲液
pH 4.3 Acetate buffer99.52 100.20 100.53 99.82 100.05 100.73 100.14±0.41 0.41 pH 6.8 磷酸缓冲液
pH 6.8 Phosphate buffer100.94 101.02 100.30 99.49 101.18 101.14 100.68±0.61 0.60 表 3 氟苯尼考在不同释放介质溶液中的稳定性试验结果
Table 3 Results of the stability test of florfenicol in different release medium solutions
释放介质
Release medium不同采样时间在标示含量中的占比/% Percentage in indicated content at different sampling time RSD/% 0 h 1 h 2 h 3 h 4 h 5 h 6 h 7 h 8 h ${\bar{\textit{} X} } \pm {\rm{SD} }$ pH 1.2 盐酸缓冲液
pH 1.2 Hydrochloric acid buffer97.33 100.12 99.00 95.05 100.56 99.51 99.40 97.91 100.84 98.86±0.02 1.86 pH 4.3 醋酸缓冲液
pH 4.3 Acetate buffer99.10 101.85 100.60 101.09 101.25 100.40 97.93 98.85 102.19 100.36±0.01 1.44 pH 6.8 磷酸缓冲液
pH 6.8 Phosphate buffer97.43 101.33 100.03 100.78 102.61 101.36 102.70 98.57 102.21 100.78±0.02 1.80 表 4 氟苯尼考缓释颗粒中药物含量的测定结果
Table 4 Determination of drug content in fluorfenicol sustained-release granules
% 批次
Batch实际含量( ${\bar{\textit{} X} } \pm {\rm{SD} }$)
Actual contentRSD 批次
Batch在标示含量中的占比( ${\bar{\textit{} X} } \pm {\rm{SD} }$)
Percentage in indicated contentRSD 1 9.89±0.09 0.93 1 99.19±0.008 0.82 2 9.95±0.08 0.85 2 100.01±0.009 0.86 3 9.72±0.08 0.87 3 97.45±0.008 0.77 4 10.02±0.04 0.42 4 100.72±0.002 0.24 表 5 氟苯尼考缓释颗粒体外累积释放方程拟合
Table 5 Fitting of cumulative release equations of florfenicol sustained-release granules
释放介质 Releasse medium 方程模型 Equation model 回归方程1) Regression equation R2 pH 1.2 盐酸缓冲液
pH 1.2 Hydrochloric acid bufferZero-order M=0.006 79t+0.014 91 0.649 49 First-order ln(1−M)=−0.007 14t−0.014 62 0.655 46 Higuchi M=0.025 12t1/2−0.004 13 0.804 98 pH 4.3 醋酸缓冲液
pH 4.3 Acetate bufferZero-order M=0.090 19t+0.442 3 0.690 03 First-order ln(1−M)=−0.771 85t−0.046 22 0.998 96 Higuchi M=0.333 68t1/2+0.189 37 0.854 28 pH 6.8 磷酸缓冲液
pH 6.8 Phosphate bufferZero-order M=0.091 21t+0.431 13 0.690 13 First-order ln(1−M)=−0.763 80t−0.030 72 0.999 32 Higuchi M=0.337 40t1/2+0.175 45 0.854 01 1) M表示累计释放百分率,t表示取样时间
1) M indicates cumulative release ratio, t indicates sampling time -
[1] 张春辉, 邢广旭, 胡骁飞, 等. 动物专用药物氟苯尼考的研究进展[J]. 中兽医医药杂志, 2019, 38(5): 95-98. [2] 周鹏, 李炜, 董国权. 氟苯尼考在动物疾病治疗中的应用效果[J]. 中国畜禽种业, 2019: 45. [3] 杨凡凡. 三种药物对猪传染性胸膜肺炎的疗效试验[J]. 贵州畜牧兽医, 2017, 41(6): 21-24. doi: 10.3969/j.issn.1007-1474.2017.06.007 [4] 李亚男, 杨帆, 王丹, 等. 氟苯尼考的药效学及其对禽源致病菌的治疗作用[J]. 广东畜牧兽医科技, 2015, 40(1): 1-4. doi: 10.3969/j.issn.1005-8567.2015.01.001 [5] 徐力文, 廖昌容, 刘广锋. 氟苯尼考用于水产养殖的安全性[J]. 中国水产科学, 2005, 12(4): 512-518. doi: 10.3321/j.issn:1005-8737.2005.04.025 [6] ZHANG Q Q, YANG G G, PAN C G, et al. Comprehensive evaluation of antibiotics emission and fate in the river basins of china: Source analysis, multimedia modeling, and linkage to bacterial resistance[J]. Environ Sci Technol, 2015, 49(11): 6772-6782. doi: 10.1021/acs.est.5b00729
[7] 魏海涛, 刘锋, 钟笑婷, 等. 水溶性氟苯尼考的研制[J]. 中国兽药杂志, 2009, 43(2): 12-15. doi: 10.3969/j.issn.1002-1280.2009.02.004 [8] 魏海涛, 宋敏, 李亮华, 等. 氟苯尼考−β−环糊精包合物的研制[J]. 华南农业大学学报, 2009, 30(4): 94-97. doi: 10.3969/j.issn.1001-411X.2009.04.022 [9] HU D, ZHANG T, ZHANG Z, et al. Toxicity to the hematopoietic and lymphoid organs of piglets treated with a therapeutic dose of florfenicol[J]. Vet Immunol Immunop, 2014, 162(3/4): 122-131.
[10] GUAN S, LU J, SHEN X. Florfenicol impairs the immune responses to vaccination against foot-and-mouth disease in mice[J]. Immunopharm Immunot, 2011, 33(4): 609-613. doi: 10.3109/08923973.2011.552507
[11] 中国兽药典委员会. 中华人民共和国兽药典[M]. 北京: 中国农业出版社, 2015: 306-308. [12] GUO B L, GAO Q Y. Preparation and properties of a pH/temperature-responsive carboxymethyl chitosan/poly (N-isopropylacrylamide) semi-IPN hydrogel for oral delivery of drugs[J]. Carbohyd Res, 2007, 342(16): 2416-2422. doi: 10.1016/j.carres.2007.07.007
[13] 陈元明, 刘金旭. 猪消化道的酸碱度(pH)[J]. 中国畜牧杂志, 1964: 5-6. [14] 谢沐风. 如何科学、客观地制订溶出度试验质量标准[J]. 中国医药工业杂志, 2012, 43(3): A23-A32. [15] 李仲谨, 田晓静, 杨威. 氟苯尼考淀粉微球的制备及缓释性能的研究[J]. 食品工业科技, 2011, 32(2): 205-207. [16] 裴艳新, 王德华. 哺乳动物消化道内食物滞留时间的影响因素[J]. 动物学杂志, 2000, 35(5): 50-53. doi: 10.3969/j.issn.0250-3263.2000.05.015 [17] 李智慧, 黄山, 孙文静, 等. 氟苯尼考肠溶缓释包衣微粒的制备及体外评价[J]. 中国兽药杂志, 2011, 45(12): 31-34. doi: 10.3969/j.issn.1002-1280.2011.12.011




 下载:
下载: