Responses of soil bacterial communities in soybean rhizosphere of abandoned mining area to fertilization methods
-
摘要:目的
从细菌多样性和组成结构角度评估化学肥料与硅酸钠或蚯蚓结合施用对废弃矿区大豆根际细菌群落的影响,为日后矿区复垦和生态恢复提供理论依据。
方法在广东省梅州市黄畲村废弃矿区种植大豆‘华春9号’,试验设置4个施肥处理:氮磷钾肥结合硅酸钠(3NPK+S和5NPK+S),氮磷钾肥结合蚯蚓20条·m−2(3NPK+E和5NPK+E),以不施肥为对照(3CK和5CK)。在施肥后3和5个月时对大豆根际土壤进行采样。通过提取土壤细菌总DNA、16S rRNA测序和测定土壤基本化学性质,探究该矿区大豆根际细菌群落多样性和结构对不同施肥处理的动态响应过程。
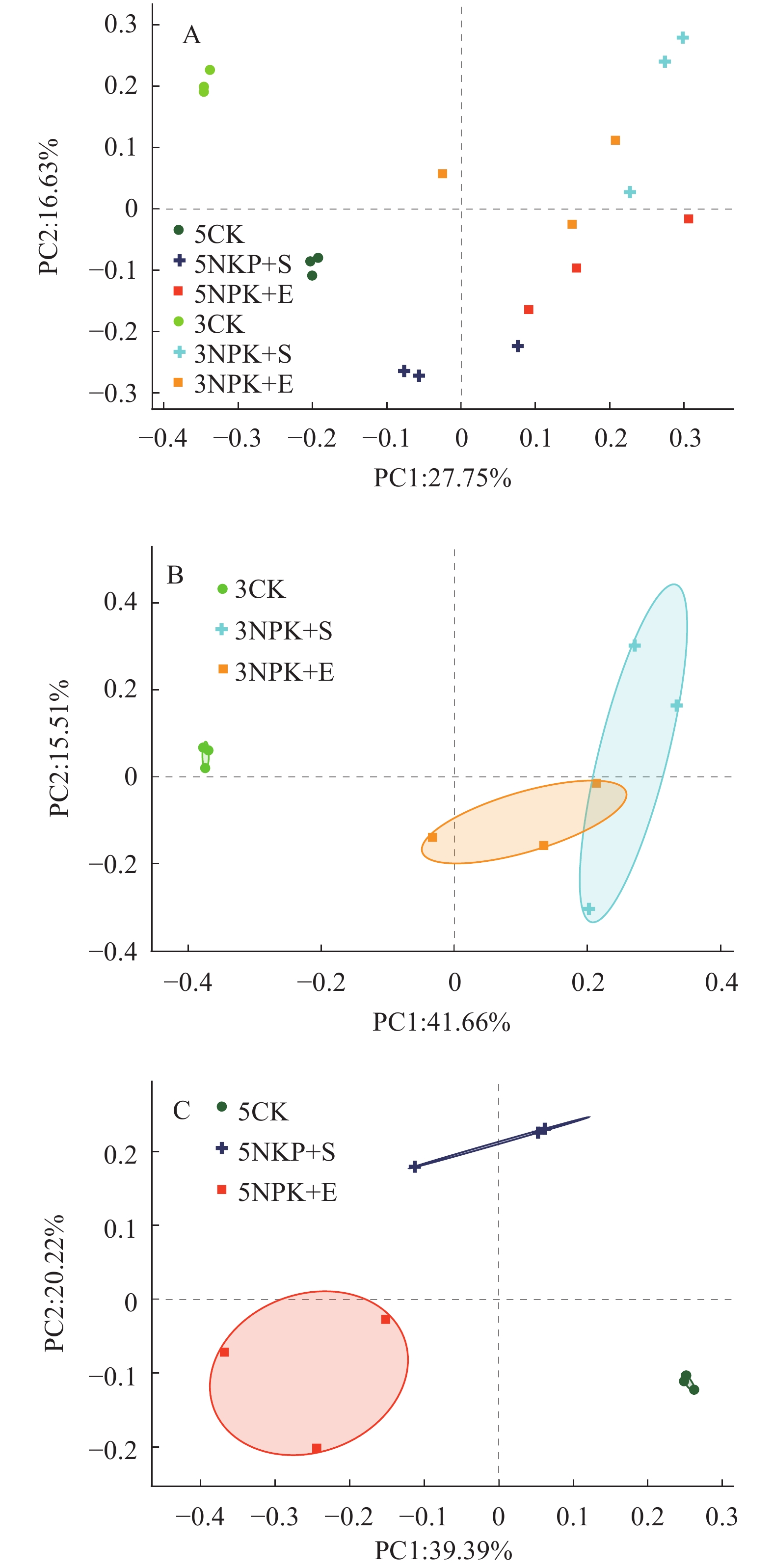
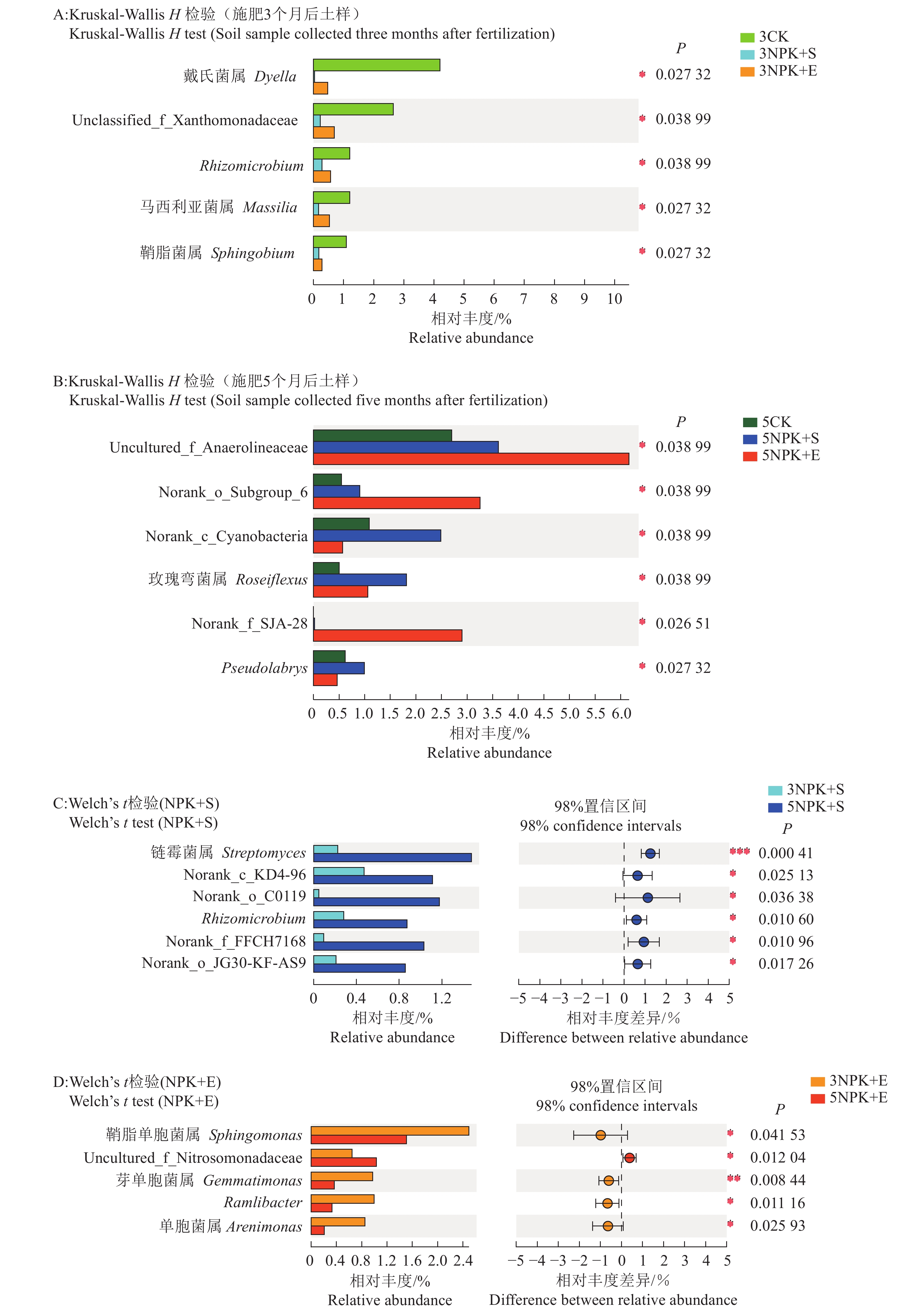
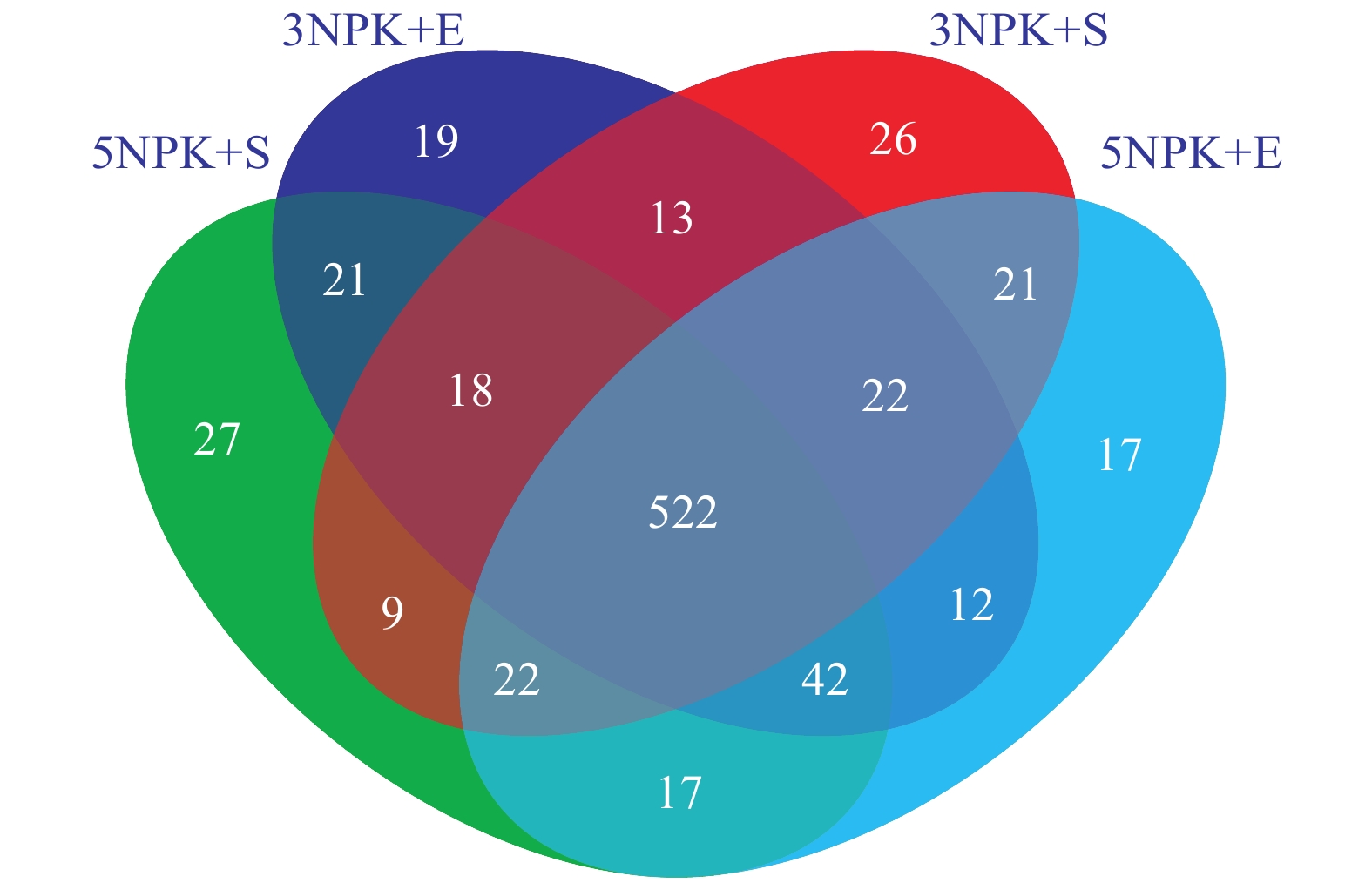
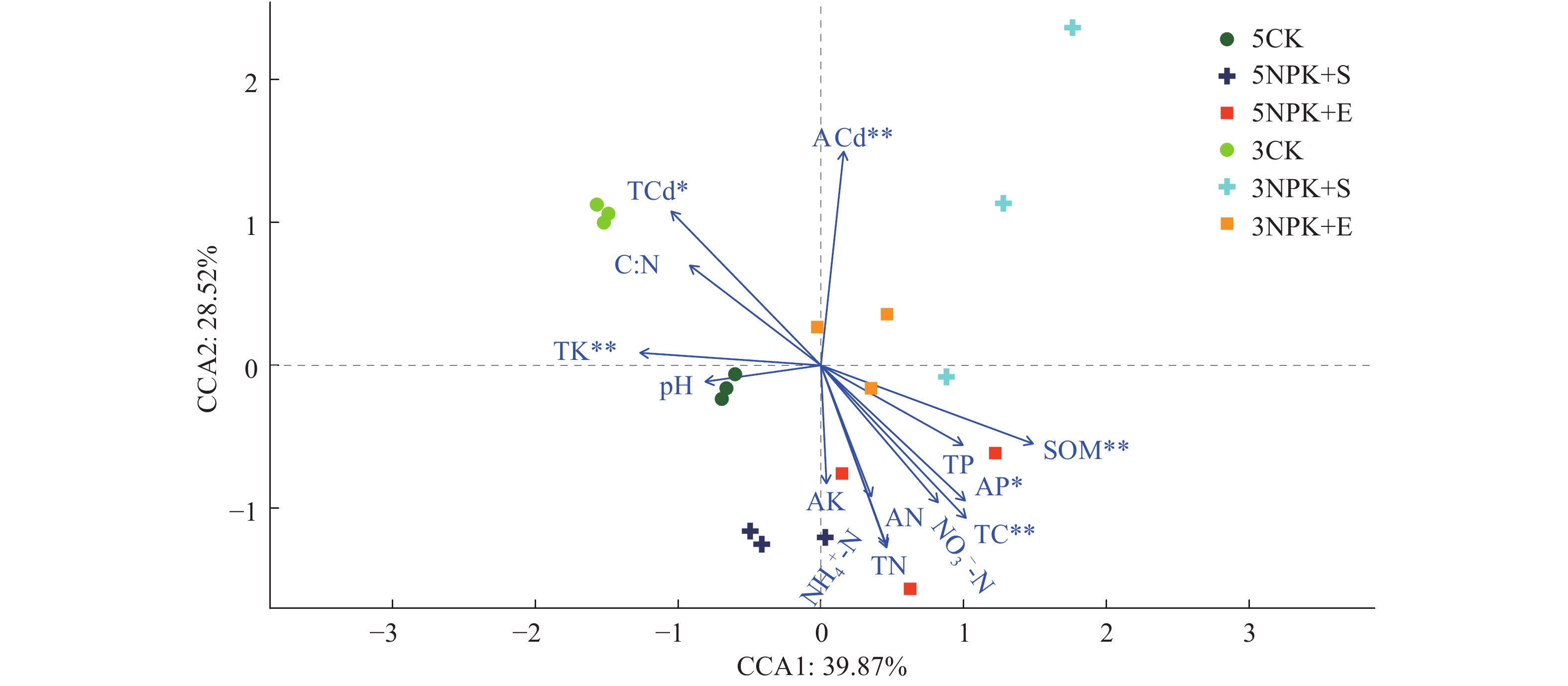
结果与3CK相比,3NPK+E处理显著增加了Chao1和Shannon指数;与5NPK+E处理相比,5NPK+S处理显著增加了Shannon指数。主坐标分析(PCoA)表明,不同处理下的根际细菌群落组成在操作分类单元(OTU)水平上出现显著分离。从属的相对丰度来看,Uncultured_f_Anaerolineaceae是3NPK+S、3NPK+E、5NPK+S、5NPK+E处理土壤中的优势菌属。基于Mantel检验和典型关联分析(CCA)显示,土壤有机质、有效磷和全碳含量对5NPK+E处理的根际细菌群落有显著影响。
结论施加NPK+S和NPK+E肥料均能有效提高矿区土壤细菌多样性,从而间接对土壤修复起促进作用。施肥方式和处理时间均会显著影响细菌群落组成,并且施肥处理时间越长对细菌群落组成的影响越明显。Uncultured_f_Anaerolineaceae富集在施肥处理下的土壤中,这类细菌可能在促使碳转化的过程中起重要作用,同时还具有修复土壤污染的潜力。NPK+E施用增加了有机质含量,对细菌群落的生长有促进作用。
Abstract:ObjectiveTo evaluate the effects of chemical fertilizers combined with sodium silicate or earthworm on bacterial community in soybean rhizosphere of abandoned mining areas from the perspectives of bacterial diversity and composition structure, and provide a theoretical basis for future mining area reclamation and ecological restoration.
Method‘Huachun 9’ soybean was planted in the abandoned mining area of Huangshe Village, Meizhou City, Guangdong, China. Four fertilization treatments were set up. Nitrogen (N), phosphorus (P) and potassium (K) fertilizers were combined with sodium silicate (3NPK+S and 5NPK+S), NPK fertilizers were combined with 20 earthworms per square metre (3NPK+E and 5NPK+E), and No fertilizer was used as control (3CK and 5CK). Soybean rhizosphere soil was sampled three and five months after fertilizing respectively. The soil bacterial total DNA was extracted, 16S rRNA was sequenced and basic soil chemical properties were determined to analyze diversity and structure of bacterial community in soybean rhizosphere responsing to different fertilization treatments in the mining area.
ResultCompared with 3CK, 3NPK+E treatment significantly increased Chao1 and Shannon indexes. Compared with 5NPK+E, 5NPK+S treatment significantly increased Shannon index. The principal coordinate analysis (PCoA) showed that rhizosphere bacterial communities in different treatments were significantly separated at operational taxonomic unit (OTU) level. In terms of relative abundance, uncultured_f_Anaerolineaceae was the dominant genus in soil treated by 3NPK+S, 3NPK+E, 5NPK+S, and 5NPK+E. Mantel test and canonical correlation analysis (CCA) showed that soil organic matter, available phosphorus and total carbon contents had significant effects on rhizosphere bacterial community of 5NPK+E treatment.
ConclusionThe applications of NPK+S and NPK+E fertilizers can effectively improve bacterial diversity, and indirectly promote soil amendment. The bacterial communities are significantly affected by different fertilization treatments. The longer the fertilization duration was, the more significant the effect was. Uncultured_f_Anaerolineaceae is enriched in fertilized soil. It may play an important role in promoting carbon transformation and has the potential to repair soil pollution. NPK+E fertilizer increases organic matter content and promotes growth of bacterial community.
-
苦楝Melia azedarach又名翠树、楝树、紫花树、森树等,为楝科楝属落叶乔木,分布于中国、韩国、日本、印度、斯里兰卡、印度尼西亚和澳大利亚等地,欧洲、美洲也有栽培[1].苦楝在我国分布广泛,水平分布为北纬18° ~ 40°,南至海南省崖县,北到河北保定和山西运城、陕西渭南、陇南地区,东至台湾、沿海各省,西到四川、云南保山[2].它生长速度快、木材材质优良、纹理美丽,易加工,可用于家具、建筑、农具、船舶、乐器制作等方面,木材抗白蚁、抗虫蛀、耐腐.苦楝耐烟尘,能大量吸收有毒有害气体,是优良的城市及工矿区绿化树种,也是我国南方四旁绿化常用树种[3-4].苦楝的根、皮、花、果均可入药,也可作为植物源农药[5].遗传多样性是生物多样性的重要组成部分,SRAP(Sequence-related amplified polymorphism,相关序列扩增多态性)结合了AFLP及RAPD各自的优点,方便快速,只需要极少量DNA材料,且不需要预先知道DNA序列信息,即可快速获得大量的信息,试验结果稳定可靠,且再现性较高,重复性较好[6-7].目前为止,国内对于苦楝的遗传多样性分析,鲜见开展过SRAP的研究.本试验采用单因素和正交试验设计从DNA、dNTPs、Mg2+、引物和TaqDNA聚合酶5个组分浓度对苦楝SRAP-PCR反应体系进行优化,旨在寻找一种高效、快速、经济的试验方法,建立适合苦楝的SRAP-PCR反应体系,为进一步应用SRAP技术对苦楝群体遗传多样性、种质资源鉴定等研究提供参考[7].
1. 材料与方法
1.1 材料
苦楝幼叶于2013年7月取自华南农业大学苗圃,随用随采,用于苦楝基因组DNA的提取,采集叶片分别为海南三亚、广东兴宁、广西梧州、福建建瓯、江西南昌、安徽利辛、陕西蒲城、河北邯郸种源.所用正向引物序列为Me19(TGAGTCCAAACCGGTTG)和Me27(TGGGGACAACCCGGCTT),反向引物序列为Em2(GACTGCGTACGAATTTGC)、Em4(GACTGCGTACGAATTTGA)和Em5(GACTGCGTACGAATTAAC).
1.2 主要试剂和仪器
用于SRAP-PCR反应的Taq酶、dNTPs、Mg2+为TaKaRa公司产品,引物由北京华大基因研究中心合成,PCR反应在东胜创新生物技术有限公司的PCR扩增仪上进行,DNA浓度和纯度使用超微量紫外分光光度计(Thermo Nanodrop 2000)检测.
1.3 基因组DNA的提取
苦楝基因组DNA提取参照上海生工生物工程有限公司柱式基因组DNA提取试剂盒说明书进行.所提取的基因组DNA用8 g·L-1琼脂糖凝胶电泳检测品质,并采用超微量紫外分光光度计检测DNA的浓度和纯度,然后将DNA稀释至50 ng·μL-1,置于-20 ℃条件下保存备用.
1.4 PCR扩增
SRAP-PCR反应程序为:94 ℃预变性5 min;94 ℃变性1 min,35 ℃复性1 min,72 ℃延伸1 min,5个循环;94 ℃变性1 min,50 ℃复性1 min,72 ℃延伸1 min,30个循环;72 ℃延伸10 min.扩增产物采用20 g·L-1的琼脂糖凝胶电泳,电泳后在自动凝胶图像分析仪上拍照分析.
1.5 PCR反应体系单因素分析
对影响苦楝SRAP-PCR反应的主要因素(模板DNA、dNTPs、Mg2+、引物和Taq酶)进行单因子试验.对各影响因子分别设置8个梯度处理:模板DNA为0、10、20、30、40、50、60和70 ng;dNTPs为0、0.05、0.10、0.15、0.20、0.25、0.30和0.35 mmol · L-1;Mg2+为0、1.0、1.5、2.0、2.5、3.0、3.5和4.0 mmol·L-1;引物为0、0.16、0.24、0.32、0.40、0.48、0.56和0.64 μmol·L-1;Taq DNA聚合酶为0、0.50、0.75、1.00、1.25、1.50、1.75和2.00 U.
1.6 PCR反应体系的正交试验
在对影响苦楝SRAP-PCR反应的模板DNA、dNTPs、Mg2+、引物和Taq酶进行单因子试验后采用L16(45)正交试验设计,共16个处理,每个处理设2个重复,各因素水平见表 1.根据电泳条带的多少、清晰度及背景颜色进行打分.最优的得5分,最差的得1分,并计算每个因素在不同水平下的平均得分[8].
表 1 SRAP-PCR正交试验设计L 16(45)及试验结果Table 1. L 16(45) Orthogonal designs and results of SRAP-PCR reaction
2. 结果与分析
2.1 单因素试验分析
以SRAP-PCR反应产物电泳得到的条带数目较多且清晰为筛选原则,对反应体系中起主要作用的5个因素进行单因素浓度梯度筛选试验[9-12],每个因素设置8个浓度梯度.试验结果表明:在25 μL反应体系中,模板DNA为25 ~ 40 ng、dNTPs为0.125 ~ 0.200 mmol·L-1、Mg2+为1.75 ~ 2.25 mmol·L-1、引物为0.40 ~ 0.52 μmol·L-1、Taq DNA聚合酶为0.50 ~ 1.25 U时扩增效果好,条带较多且清晰,故将其选为后续正交试验的适宜浓度范围.
2.2 苦楝SRAP-PCR正交反应体系的优化
以上述单因素试验确定的各因素适宜浓度范围为基础,采用L 16(45)正交设计对SRAP-PCR反应体系进行优化(表 1),并根据电泳条带的多少、清晰度及背景颜色(图 1)对16个处理进行打分,打分结果如表 1所示,从2次的得分来看,重复间差异不大,试验的一致性较好,其中处理5、处理7、处理8和处理9效果较好,评分均为4分,而处理15效果不好,评分仅为1.0分.从图 2可见,模板DNA 30 ng、dNTPs 0.125 mmol·L-1、Mg2+ 2.25 mmol·L-1、引物0.48 μmol·L-1、Taq DNA聚合酶0.75 U、反应总体积25 μL时得分较高,实现最佳扩增,确定为最优组合.
2.3 苦楝SRAP-PCR反应体系稳定性的检测
为了验证体系的准确性,以来自海南三亚、广东兴宁、广西梧州、福建建瓯、江西南昌、安徽利辛、陕西蒲城、河北邯郸的8个苦楝种源DNA为模板,选取引物Me27/Em2、Me27/Em4进行SRAP-PCR验证,其结果如图 3所示,每个种源对每个引物均有清晰的条带,且不同种源间条带有差异.由此可见,本试验建立的SRAP-PCR体系稳定可靠,适用于苦楝后续的SRAP分析.
3. 结论
本试验建立并优化了适应苦楝SRAP-PCR的反应体系,前期对苦楝模板DNA、Mg2+、引物和Taq酶进行单因子试验,研究发现,SRAP对苦楝DNA浓度的要求不高,有一个较宽的浓度适宜范围,在25 μL体系中,模板DNA为10 ~ 70 ng时都扩增出了较清晰、带型基本相同的谱带;dNTPs设计的8个浓度梯度中,0.1 ~ 0.2 mmol·L-1范围内能扩增出清晰谱带,且条带基本相同,浓度低于0.1 mmol·L-1时,扩增条带弥散,高于0.2 mmol·L-1时,出现条带丢失的现象;Mg2+为2.00 mmol·L-1左右时扩增条带较清晰且数量多;引物介于0.48 ~ 0.64 μmol·L-1之间均能产生较为清晰的条带,且带型基本上保持一致,条带数并没有随着浓度的增加而增加;Taq DNA聚合酶用量在0.50 ~ 2.0 U范围内均可以得到清晰的带型,对其用量要求不高.进一步对苦楝SRAP-PCR的反应体系进行正交试验,并根据电泳条带的多少、清晰度及背景颜色对16个处理进行打分,从2次的得分来看,重复间差异不大,试验的一致性较好,其中处理5、处理7、处理8和处理9效果较好,评分均为4.0分,而处理15效果不好,评分仅为1.0分.根据得分可知,在25 μL反应体系中,当模板DNA 30 ng、dNTPs 0.125 mmol·L-1、Mg2+ 2.25 mmol·L-1、引物0.48 μmol·L-1、Taq DNA聚合酶0.75 U时,实现最佳扩增,确定为最优组合.以来自海南三亚、广东兴宁、广西梧州、福建建瓯、江西南昌、安徽利辛、陕西蒲城、河北邯郸的8个苦楝种源DNA为模板,选取引物Me27/Em2、Me27/Em4进行SRAP-PCR反应体系稳定性验证,结果表明,筛选体系能很好地满足苦楝基因组SRAP-PCR扩增的要求且不同种源间条带有差异.
-
图 5 基于CCA分析的根际细菌群落与土壤化学性质之间的关系
SOM:土壤有机质,TC:全碳,TN:全氮,C︰N:碳氮质量比,TP:全磷,TK:全钾,AN:碱解氮,AP:有效磷,AK:速效钾,TCd:全镉,ACd:速效镉,NH4+-N:铵态氮,NO3--N:硝态氮;“*”和“**”分别表示差异达0.05和0.01显著水平(Mantel检验)
Figure 5. Correlation between rhizosphere bacterial community and soil chemical property based on CCA analysis
SOM: Soil organic matter, TC: Total carbon, TN: Total nitrogen, C︰N: C︰N mass ratio, TP: Total phosphorus, TK: Total potassium, AN: Alkaline nitrogen, AP: Available phosphorus, AK: Available potassium, TCd: Total cadmium, ACd: Available cadmium, NH4+-N: Ammonium nitrogen, NO3--N: Nitrate nitrogen; “*” and “**” indicated significant differences at 0.05 and 0.01 levels respectively (Mantel test)
表 1 根际土壤化学性质1)
Table 1 Chemical properties of rhizosphere soil
处理
Treatment取样时间/月
Sampling timepH w/(g·kg−1) C︰N质量比
C︰N mass ratioSOM TC TN TP TK 3CK 3 5.60±0.20a 0.67±0.12b 4.44±0.34c 0.05±0.01b 0.04±0.01b 60.43±0.84a 85.22±14.23a 3NPK+S 3 4.75±0.04b 4.00±0.89a 7.45±0.95b 0.16±0.04a 0.08±0.02a 41.11±6.62b 47.11±6.90b 3NPK+E 3 4.74±0.20b 3.75±0.58a 11.56±0.56a 0.20±0.02a 0.07±0.02a 47.77±5.34b 57.41±8.71b 5CK 5 5.40±0.35a 0.70±0.10c 5.77±0.74c 0.05±0.01b 0.07±0.05a 60.43±0.84a 114.25±16.67a 5NPK+S 5 4.71±0.21b 3.52±1.04b 10.62±1.50b 0.69±0.16a 0.07±0.02a 41.11±6.62b 15.87±3.80b 5NPK+E 5 5.67±0.21a 5.78±0.30a 15.98±0.57a 0.48±0.16a 0.12±0.02a 50.08±8.12ab 36.35±14.97b 处理
Treatment取样时间/月
Sampling timew/(mg·kg−1) AN AP AK TCd ACd NH4+-N NO3−-N 3CK 3 16.74±1.33b 4.37±0.21c 32.33±1.00a 0.14±0.02a 0.02±0.00a 31.35±1.43b 1.94±0.26b 3NPK+S 3 19.72±1.89ab 10.2±1.13a 34.38±1.48a 0.08±0.01b 0.02±0.00a 36.06±0.66a 2.91±0.29a 3NPK+E 3 20.77±1.44a 7.77±0.03b 32.55±0.70a 0.06±0.00b 0.01±0.00b 37.76±0.41a 2.59±0.51ab 5CK 5 16.07±0.36b 5.10±0.26c 34.99±1.53b 0.09±0.01a 0.01±0.00a 34.99±4.51b 3.02±0.63a 5NPK+S 5 35.07±1.61a 13.99±2.08a 54.96±9.02a 0.05±0.02a 0.01±0.01a 41.65±2.89a 4.39±1.06a 5NPK+E 5 30.63±5.16a 9.43±0.84b 31.75±9.14b 0.06±0.02a 0.01±0.00a 37.73±1.50ab 2.84±0.87a 1)同列数据后的不同小写字母表示相同取样时间的处理间差异显著(P<0.05,Duncan’s法);SOM:土壤有机质,TC:全碳,TN:全氮,TP:全磷,TK:全钾,AN:碱解氮,AP:有效磷,AK:速效钾,TCd:全镉,ACd:速效镉,NH4+-N:铵态氮,NO3−-N:硝态氮
1) Different lowercase letters in the same column indicate significant differences among different treatments of the same sampling time (P< 0.05, Duncan’s method); SOM: Soil organic matter, TC:Total carbon, TN: Total nitrogen, TP: Total phosphorus, TK: Total potassium, AN: Alkaline nitrogen, AP: Available phosphorus, AK: Available potassium, TCd: Total cadmium, ACd: Available cadmium, NH4+-N: Ammonium nitrogen, NO3−-N: Nitrate nitrogen表 2 根际土壤样品的细菌群落多样性指数1)
Table 2 Indexes of bacterial community diversity in rhizosphere soil samples
处理
Treatment取样时间/月
Sampling timeChao1指数
Chao1 richness estimatorShannon指数
Shannon diversity index3CK 3 2 208.78±87.56b 5.63±0.04c 3NPK+S 3 2 429.35±324.20b 6.03±0.14b 3NPK+E 3 3 051.00±343.02a 6.43±0.20a 5CK 5 3 041.49±97.74a 6.45±0.11ab 5NPK+S 5 3 414.47±53.51a 6.68±0.06a 5NPK+E 5 3 099.13±354.25a 6.22±0.14b 1)同列数据后的不同小写字母表示相同取样时间处理间差异显著(P<0.05,Duncan’ s法)
1) Different lowercase letters within the same column indicate significant differences among different treatments of the same sampling time (P< 0.05, Duncan’ s method)表 3 不同施肥处理下根际土壤优势菌门的相对丰度1)
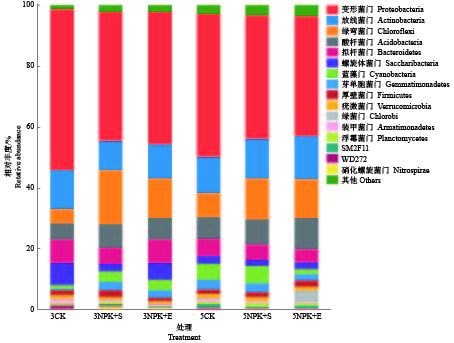
Table 3 Relative abundance of rhizosphere soil dominant bacteria at phylum level under different fertilization treatments
处理
Treatment取样时间/月
Sampling time相对丰度/%
Relative abundance变形菌门
Proteobacteria放线菌门
Actinobacteria绿弯菌门
Chloroflexi酸杆菌门
Acidobacteria拟杆菌门
Bacteroidetes3CK 3 52.74±1.40a 12.55±2.02a 4.76±0.87b 5.35±0.65a 7.82±1.28a 3NPK+S 3 42.70±10.53a 9.60±2.16a 17.58±7.72a 7.94±2.07a 5.21±2.27a 3NPK+E 3 43.18±4.49a 11.14±1.95a 13.32±3.71ab 7.28±4.38a 7.59±0.94a 5CK 5 47.06±0.58a 11.93±0.80a 8.08±1.26b 6.89±0.95a 5.71±0.42a 5NPK+S 5 40.64±2.27b 12.93±1.07a 13.67±0.66a 8.17±1.45a 5.11±0.40a 5NPK+E 5 39.70±2.27b 14.47±3.27a 13.01±0.79a 10.45±2.30a 4.46±0.58a 1)同列数据后的不同小写字母表示相同取样时间处理间差异显著(P<0.05,Duncan’s法)
1) Different lowercase letters within the same column indicate significant difference among different treatments of the same sampling time (P<0.05, Duncan’s method)表 4 不同施肥处理下根际土壤优势菌属的相对丰度1)
Table 4 Relative abundance of rhizosphere soil dominant bacteria at genus level under different fertilization treatments
处理
Treatment取样时间/月
Sampling time相对丰度/% Relative abundance Uncultured_f_Anaerolineaceae Norank_p_Saccharibacteria 慢生根瘤菌属
Bradyrhizobium鞘氨醇单胞菌属
Sphingomonas伯克氏菌属
Burkholderia3CK 3 1.43±0.86b 7.41±1.57a 1.07±0.12a 1.46±0.12a 6.36±2.23a 3NPK+S 3 10.49±4.80a 2.47±0.69b 1.38±1.23a 1.55±0.73a 0.51±0.54b 3NPK+E 3 7.46±2.80ab 5.34±2.38ab 1.26±0.26a 2.45±0.49a 1.41±1.11b 5CK 5 2.75±0.79b 2.56±0.27a 2.87±0.52a 2.28±0.39ab 1.16±0.10a 5NPK+S 5 3.65±0.66b 2.08±0.36a 2.37±0.28a 2.45±0.14a 1.00±0.33a 5NPK+E 5 6.22±2.11a 2.27±0.20a 3.22±2.10a 1.52±0.30b 0.40±0.13b 1)同列数据后的不同小写字母表示相同取样时间处理间差异显著(P<0.05,Duncan’ s法)
1) Different lowercase letters within the same column indicate significant difference among different treatments of the same sampling time (P< 0.05, Duncan’ s method) -
[1] 王娟, 王正海, 耿欣, 等. 大宝山多金属矿区土壤–植被稀土元素生物地球化学特征[J]. 中国地质大学学报(地球科学版), 2014, 39(6): 733-740. [2] ZHAO J, NI T, LI Y, et al. Responses of bacterial communities in arable soils in a rice-wheat cropping system to different fertilizer regimes and sampling times[J/OL]. PLoS One, 2014, 9(1), e85301[2019-06-10]. https://doi.org/10.1371/journal.pone.0085301.
[3] NANNIPIERI P, ASCHER J, CECCHERINI MT, et al. Microbial diversity and soil functions[J]. Eur J Soil Sci, 2003, 54(4): 655-670. doi: 10.1046/j.1351-0754.2003.0556.x
[4] 吴国伟, 赵艳玲, 付艳华, 等. 复垦矿区土地利用类型变化对植被碳储量的影响[J]. 中国生态农业学报, 2015, 23(11): 1437-1444. [5] 刘杏兰, 高宗, 刘存寿, 等. 有机−无机肥配施的增产效应及对土壤肥力影响的定位研究[J]. 土壤学报, 1996(2): 138-147. doi: 10.11766/trxb199407060204 [6] CLEMENTE R, PAREDES C, BERNAL M P, et al. A field experiment investigating the effects of olive husk and cow manure on heavy metal availability in a contaminated calcareous soil from Murcia (Spain)[J]. Agric Ecosyst Environ, 2007, 118(1/2/3/4): 319-326.
[7] LI X, RUI J, MAO Y, et al. Dynamics of the bacterial community structure in the rhizosphere of a maize cultivar[J]. Soil Biol Biochem, 2014, 68: 392-401. doi: 10.1016/j.soilbio.2013.10.017
[8] DIMKPA C, WEINAND T, ASCH F. Plant-rhizobacteria interactions alleviate abiotic stress conditions[J]. Plant Cell Environ, 2009, 32(12): 1682-1694. doi: 10.1111/j.1365-3040.2009.02028.x
[9] 王梦姣, 杨国鹏, 乔帅, 等. 植物−根际微生物协同修复有机物污染土壤的研究进展[J]. 江苏农业科学, 2017, 45(1): 5-8. [10] LAMBERT D H, WEIDENSAUL F C. Element uptake by myeorrhizal soybean from sewage-sludge-treated soil[J]. Soil Sci Soc Am J, 1991, 55(2): 393-398.
[11] 李建华, 郜春花, 卢朝东, 等. 丛枝菌根和根瘤菌双接种对矿区土地复垦的生态效应[J]. 中国土壤与肥料, 2009(5): 77-80. [12] DONG R, GU L, GUO C, et al. Effect of PGPR Serratiamarcescens BC-3 and AMF glomusintraradices on phytoremediation of petroleum contaminated soil[J]. Ecotoxicology, 2014, 23(4): 674-680. doi: 10.1007/s10646-014-1200-3
[13] 杨海君, 肖启明, 刘安元. 土壤微生物多样性及其作用研究进展[J]. 南华大学学报(自然科学版), 2005, 19(4): 21-31. [14] 范继香, 郜春花, 张强, 等. 施肥措施对矿区复垦土壤活性有机碳库的影响[J]. 中国农学通报, 2012, 28(36): 119-123. doi: 10.3969/j.issn.1000-6850.2012.36.020 [15] 邓晓霞, 黎其万, 米艳华, 等. 云南个旧矿区Pb污染稻田土壤钝化修复[J]. 环境工程学报, 2017, 11(8): 4831-4837. doi: 10.12030/j.cjee.201606017 [16] 张池, 周波, 吴家龙, 等. 蚯蚓在我国南方土壤修复中的应用[J]. 生物多样性, 2018, 26(10): 1091-1102. doi: 10.17520/biods.2018151 [17] 张变华, 靳东升, 张强, 等. 不同施肥处理下工矿复垦区大豆根际效应分析[J]. 大豆科学, 2018, 37(6): 93-100. [18] NECHITAYLO T Y, YAKIMOV M M, GODINHO M, et al. Effect of the earthworms Lumbricus terrestris and Aporrectodea caliginosa on bacterial diversity in soil[J]. Microb Ecol, 2010, 59(3): 574-587.
[19] 孟庆英, 于忠和, 贾绘彬, 等. 不同施肥处理对大豆根际土壤微生物及土壤肥力影响[J]. 大豆科学, 2011, 30(3): 471-474. [20] BASKER A, KIRKMAN J H, MACGREGOR A N. Changes in potassium availability and other soil properties due to soil ingestion by earthworms[J]. Biol Fert Soil, 1994, 17(2): 154-157.
[21] YU X, CHENG J, WONG M H. Earthworm-mycorrhiza interaction on Cd uptake and growth of ryegrass[J]. Soil Biol Biochem, 2005, 37(2): 195-201. doi: 10.1016/j.soilbio.2004.07.029
[22] 庞宇飞. 蚯蚓粪对城镇污泥降解过程中理化指标及微生物群落结构的影响[D]. 兰州: 兰州交通大学, 2018. [23] 刘胜洪, 周玲艳, 杨妙贤, 等. 十种耐逆植物在和平县稀土矿区生态修复中的应用[J]. 天津农业科学, 2013, 19(7): 92-96. doi: 10.3969/j.issn.1006-6500.2013.07.023 [24] 王崇臣, 王鹏, 黄忠臣. 盆栽玉米和大豆对铅、镉的富集作用研究[J]. 安徽农业科学, 2008, 36(24): 10383. doi: 10.3969/j.issn.2095-0446.2015.10.129 [25] 鲍士旦. 土壤农化分析[M]. 北京: 中国农业出版社, 1982: 25-114. [26] LIAN T, MA Q, SHI Q, et al. High aluminum stress drives different rhizosphere soil enzyme activities and bacterial community structure between aluminum-tolerant and aluminum-sensitive soybean genotypes[J]. Plant Soil, 2019, 440(1/2): 409-425.
[27] CASTRILLO G, TEIXEIRA P J P L, PAREDES S H, et al. Root microbiota drive direct integration of phosphate stress and immunity[J]. Nature, 2017, 543(7646): 513-518. doi: 10.1038/nature21417
[28] LI J, LIN J, PEI C, et al. Variation of soil bacterial communities along a chronosequence of Eucalyptus plantation[J/OL]. Peer J, 2018, 6:e5648[2019-06-11].https://doi.rog/10.7717/peerj.5648.
[29] LI L, TILMAN D, LAMBERS H, et al. Plant diversity and overyielding: Insights from belowground facilitation of intercropping in agriculture[J]. New Phytolog, 2014, 203(1): 63-69. doi: 10.1111/nph.12778
[30] SUN R, ZHANG X X, GUO X, et al. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw[J]. Soil Biol Biochem, 2015, 88(5): 9-18. doi: 10.1016/j.soilbio.2015.05.007
[31] 郝月崎, 孙扬, 李晓晶, 等. 赤子爱胜蚓对乙草胺污染土壤微生物群落的影响[J]. 农业环境科学学报, 2018, 37(11): 2456-2466. doi: 10.11654/jaes.2018-0504 [32] 陈来红, 乔光华, 董红丽, 等. 准格尔露天矿区复垦对土壤细菌多样性的影响研究[J]. 干旱区资源与环境, 2012, 26(2): 119-125. [33] 单世平, 郭照辉, 付祖姣, 等. 降低水稻镉吸收原位钝化修复技术及其作用机理[J]. 生态科学, 2015, 34(4): 175-179. [34] 李淑仪, 林翠兰, 许建光, 等. 施硅对污染土壤中铬形态及其生物有效性的影响[J]. 生态环境, 2008, 17(1): 227-231. [35] 刘晶鑫, 迟凤琴, 许修宏, 等. 长期施肥对农田黑土微生物群落功能多样性的影响[J]. 应用生态学报, 2015, 26(10): 3066-3072. [36] ZHOU X, SHEN Y, FU X, et al. Application of sodium silicate enhances cucumber resistance to fusarium wilt and alters soil microbial communities[J]. Front Plant Sci, 2018, 9: 624. doi: 10.3389/fpls.2018.00624
[37] KENNEDY A C, SMITH K L. Soil microbial diversity and the sustainability of agricultural soils[J]. Plant Soil, 1995, 170(1): 75-86. doi: 10.1007/BF02183056
[38] 于冰, 宋阿琳, 李冬初, 等. 长期施用有机和无机肥对红壤微生物群落特征及功能的影响[J]. 中国土壤与肥料, 2017(6): 58-65. doi: 10.11838/sfsc.20170609 [39] ENAMI Y, OKANO S, YADA H, et al. Influence of earthworm activity and rice straw application on the soil microbial community structure analyzed by PLFA pattern[J]. Eur J Soil Biol, 2001, 37(4): 269-272. doi: 10.1016/S1164-5563(01)01096-2
[40] YU Z H, WANG G H, JIN J, et al. Soil microbial communities are affected more by land use than seasonal variation in restored grassland and cultivated mollisols in Northeast China[J]. Eur J Soil Biol, 2011, 47(6): 357-363. doi: 10.1016/j.ejsobi.2011.09.001
[41] LIANG B, WANG L Y, ZHOU Z C, et al. High frequency of Thermodesulfovibrio spp. and Anaerolineaceae in association with Methanoculleus spp. in a long-term incubation of n-alkanes-degrading methanogenic enrichment culture[J]. Front Microb, 2016, 7: 1431. doi: 10.3389/fmicb.2016.01431
[42] 蔡萍萍, 宁卓, 何泽, 等. 采油井场土壤微生物群落结构分布[J]. 环境科学, 2018, 39(7): 3329-3338. [43] LIAN T, JIN J, WANG G, et al. The fate of soybean residue-carbon links to changes of bacterial community composition in mollisols differing in soil organic carbon[J]. Soil Biol Biochem, 2017, 109: 50-58. doi: 10.1016/j.soilbio.2017.01.026
[44] CHAOUI H I, ZIBILSKE L M, OHNO T. Effects of earthworm casts and compost on soil microbial activity and plant nutrient availability[J]. Soil Biol Biochem, 2003, 35(2): 295-302. doi: 10.1016/S0038-0717(02)00279-1
[45] HORNER-DEVINE M C, CARNEY K M, BOHANNAN B J. An ecological perspective on bacterial biodiversity[J]. Biol Sci, 2004, 271(1535): 113-122.
[46] SINGH K P, SARKAR M C. Phosphorus availability in soils as affected by fertilizer phosphorus, sodium silicate and farmyard manure[J]. Indian J Agr Sci, 1992, 40(4): 762-767.
[47] BOSSUYT H, SIX J, HENDRIX P F. Protection of soil carbon by microaggregates within earthworm casts[J]. Soil Biol Biochem, 2005, 37(2): 251-258. doi: 10.1016/j.soilbio.2004.07.035
[48] OYEDELE D J, SCHJØNNING P, AMUSAN A A. Physicochemical properties of earthworm casts and uningested parent soil from selected sites in Southwestern Nigeria[J]. Ecol Eng, 2006, 28(2): 106-113. doi: 10.1016/j.ecoleng.2006.05.002
-
期刊类型引用(0)
其他类型引用(4)



 下载:
下载: