Pathogenesis of Singapore grouper iridovirus (SGIV) and its immune control strategies
-
摘要:
虹彩病毒(Iridovirus)是目前海水和淡水养殖鱼类最严重的病毒性病原之一,已从100多种鱼类中分离鉴定出该病毒。石斑鱼虹彩病毒(Singapore grouper iridovirus, SGIV)是从新加坡养殖的患病石斑鱼中分离鉴定的高致病性虹彩病毒,是虹彩病毒科Iridoviridae蛙病毒属Iridovirus 1种新的病毒。本文从以下几个方面对SGIV的研究进行综述:SGIV的形态、超微结构及其在石斑鱼细胞中的复制和装配过程;SGIV病毒基因组、转录组、囊膜蛋白质组及miRNAs的解析;SGIV感染宿主靶标组织的鉴定;病毒侵染的入侵方式、运动轨迹和胞内运输的实时追踪;SGIV感染宿主引起类凋亡的死亡机制及介导的信号通路的揭示;多种宿主免疫抗病基因对病毒感染的调节作用;多种SGIV的检测技术,包括基于抗体的流式细胞技术、微流控芯片检测技术、环介导等温扩增技术及核酸适配体检测方法等;SGIV的灭活疫苗、亚单位疫苗和DNA疫苗的研制等。以期为深入阐明SGIV感染致病机理奠定坚实的理论基础,为发展抗病毒对策提供技术支撑。
Abstract:Iridovirus is one of the most serious viral pathogens in marine and freshwater cultured fish. Up to now, iridoviruses have been isolated and identified from more than 100 fish species worldwide. Singapore grouper iridovirus (SGIV), a novel species of Ranavirus, was isolated from diseased grouper in Singapore. On the basis of establishing a virus-sensitive cell infection model, morphology, ultrastructure, replication and biochemical characterization of SGIV in grouper host cells were studied by electron microscopy and biochemical analysis. The molecular biological characterizations of SGIV, including viral genome, transcriptome, envelope proteome and viral microRNAs, were systematically analyzed by Omics analysis. The interactions between SGIV and host were investigated from many aspects, including identifying the target tissues of SGIV infection, tracking the single virus entry and transport, non-apoptosis cell death induced by SGIV infection, functions of host immune related genes in virus infection. Meanwhile, a variety of SGIV detection technologies have been developed, including antibody-based flow cytometry, microfluidic chip detection technology platform system, loop-mediated isothermal amplification (LAMP) and nucleic acid aptamer detection method. In addition, SGIV inactivated vaccine, subunit vaccine and DNA vaccine were developed. The results provide a theoretical basis for better understanding of the pathogenic mechanism of SGIV infection, and offer technical supports for the prevention and control of SGIV.
-
-
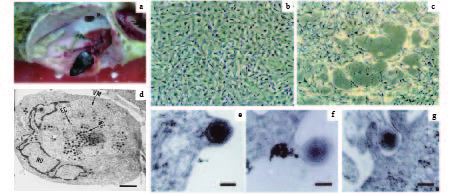
图 1 SGIV病毒的分离、纯化及细胞内的复制增殖[3, 10-11]
a:患病石斑鱼肿大的脾脏;b:正常的石斑鱼脾细胞;c:SGIV感染引起脾细胞明显的细胞病变;d:感染细胞的超微结果观察,VM:病毒装配区,EC:空衣壳,MC:成熟核衣壳,NU:细胞核,标尺1 μm;e~g分别表示SGIV病毒粒子的出芽、释放及重感染邻近细胞,标尺为100 nm
Figure 1. Isolation and purification of SGIV and its replication in cells
a: The enlarged spleen of diseased grouper; b: Mock grouper spleen cells; c: The cytopathic effects induced by SGIV infection; d: Ultrastructural observation of infected cells, VM: viromatrix, EC: empty capsid, MC: matured nucleocapsid, NU: nuclesus, scale bar is 1 μm; e~g indicate budding, release and reinfection of SGIV particles respectively, scale bars are 100 nm
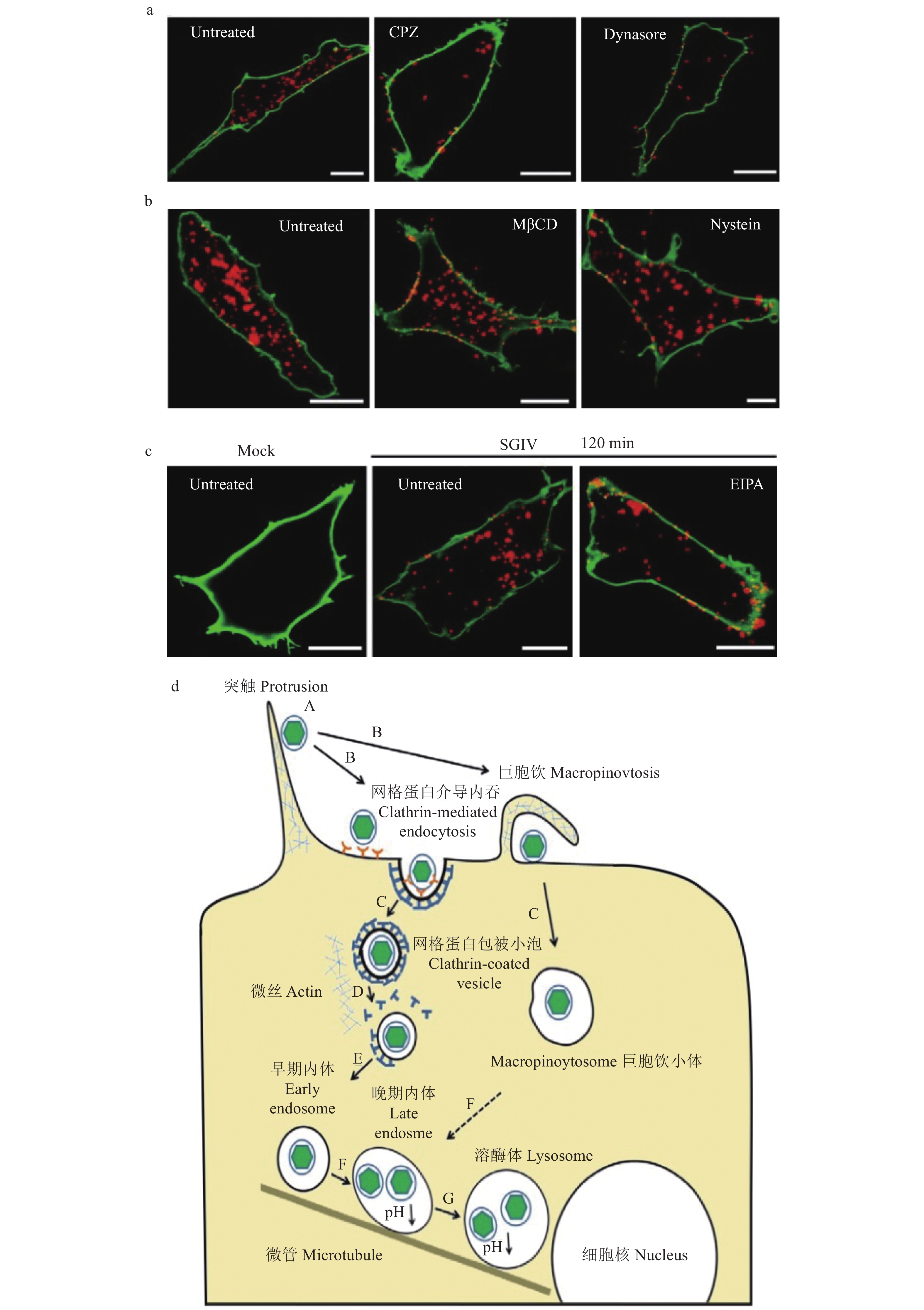
图 2 SGIV病毒进入介导的内吞方式[32]
a~c:SGIV病毒进入依赖于网格蛋白和巨胞饮,而不依赖于小窝蛋白,标尺为10 μm;d:SGIV进入细胞的模式图
Figure 2. The endocytosis mediated by SGIV entry
a~c: SGIV enters grouper cells via the clathrin-mediated endocytic pathway and micropinocytosis, but not via caveola-dependent endocytosis, scale bars are 10 μm; d: Model entry route of SGIV into GS cells
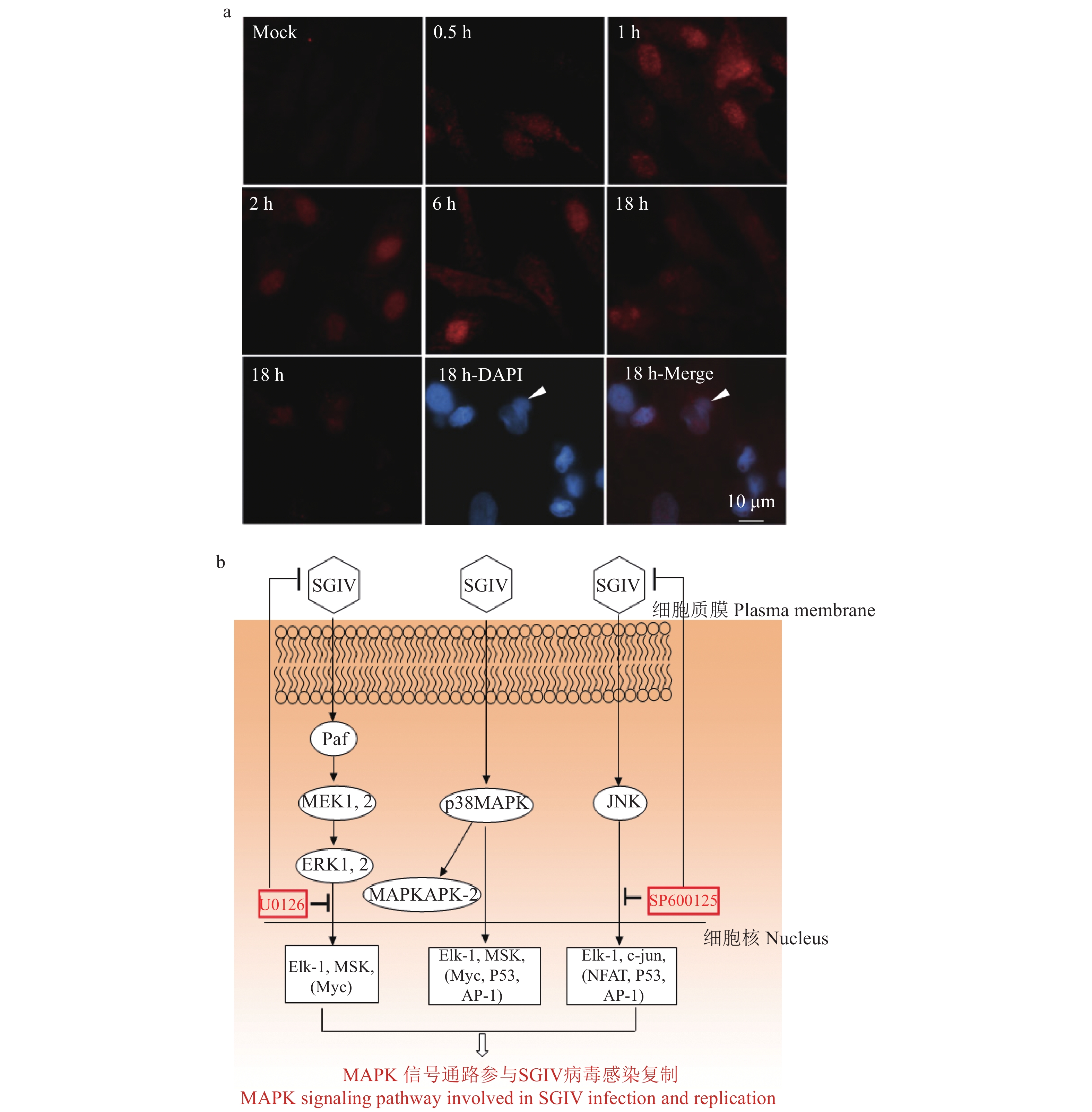
图 3 SGIV能显著激活MAPK信号通路中关键的信号分子[34, 36]
a:SGIV能激活ERK的磷酸化;b:SGIV感染对MAPK信号通路的激活情况及MAPK信号通路在病毒感染过程中的作用模式图,SGIV:新加坡石斑鱼虹彩病毒,MAPK:丝裂原活化蛋白激酶,MEK:MAPK激酶,ERK:细胞外调节蛋白激酶,JNK:c-Jun氨基末端激酶,MAPKAPK:MAPK激活蛋白激酶
Figure 3. SGIV infection significantly activates the key molecules in the MAPK signaling pathway
a: SGIV induces phosphorylation of ERK; b: Activation of MAPK signaling pathway induced by SGIV infection and the action of MAPK signaling pathway during virus infection, SGIV: Singapore grouper iridovirus, MAPK: mitogen-activated protein kinase, MEK: MAPK kinase, ERK: extracellular regulated protein kinases, JNK:c-Jun amino-terminal kinase, MAPKAPK: MAPK-activated protein kinase
-
[1] GRAY M J, CHINCHAR V G. Ranaviruses: Lethal pathogens of ectothermic vertebrates[M]. SpringerOpen, 2015.
[2] CHINCHAR V G, WALTZEK T B, SUBRAMANIAM K. Ranaviruses and other members of the family Iridoviridae: Their place in the virosphere[J]. Virology, 2017, 511: 259-271. doi: 10.1016/j.virol.2017.06.007
[3] QIN Q W, WU T H, JIA T L, et al. Development and characterization of a new tropical marine fish cell line from grouper, Epinephelus coioides susceptible to iridovirus and nodavirus[J]. J Virol Methods, 2006, 131(1): 58-64. doi: 10.1016/j.jviromet.2005.07.009
[4] HUANG X H, HUANG Y H, SUN J, et al. Characterization of two grouper Epinephelus akaara cell lines: Application to studies of Singapore grouper iridovirus (SGIV) propagation and virus-host interaction[J]. Aquaculture, 2009, 292(3): 172-179.
[5] OUYANG Z L, HUANG X H, HUANG E Y, et al. Establishment and characterization of a new marine fish cell line derived from red-spotted grouper Epinephelus akaara[J]. J Fish Biol, 2010, 77(5): 1083-1095. doi: 10.1111/jfb.2010.77.issue-5
[6] GOGN J, HUANG Y H, HUANG X H, et al. Establishment and characterization of a new cell line derived from kidney of grouper, Epinephelus akaara (Temminck & Schlegel), susceptible to Singapore grouper iridovirus (SGIV)[J]. J Fish Dis, 2011, 34(9): 677-686. doi: 10.1111/jfd.2011.34.issue-9
[7] ZHOU L L, LI P F, LIU J X, et al. Establishment and characterization of a mid-kidney cell line derived from golden pompano Trachinotus ovatus, a new cell model for virus pathogenesis and toxicology studies[J]. In Vitro Cell Dev-An, 2017, 53(4): 320-327. doi: 10.1007/s11626-016-0112-3
[8] LI P F, ZHOU L L, NI S W, et al. Establishment and characterization of a novel cell line from the brain of golden pompano (Trachinotus ovatus)[J]. In Vitro Cell Dev-An, 2016, 52(4): 410-418. doi: 10.1007/s11626-015-9988-6
[9] WEI S N, YU Y P, QIN Q W. Establishment of a new fish cell line from the caudal fin of golden pompano Trachinotus ovatus and its susceptibility to iridovirus[J]. J Fish Biol, 2018, 92(6): 1675-1686. doi: 10.1111/jfb.2018.92.issue-6
[10] QIN Q W, LAM T J, SIN Y M, et al. Electron microscopic observations of a marine fish iridovirus isolated from brown-spotted grouper, Epinephelus tauvina[J]. J Virol Methods, 2001, 98(1): 17-24. doi: 10.1016/S0166-0934(01)00350-0
[11] QIN Q W, CHANG S F, NGOH-LIM G H, et al. Characterization of a novel ranavirus isolated from grouperEpinephelus tauvina[J]. Dis Aquat Organ, 2003, 53(1): 1-9.
[12] SONG W J, QIN Q W, QIU J, et al. Functional genomics analysis of Singapore grouper iridovirus: Complete sequence determination and proteomic analysis[J]. J Virol, 2004, 78(22): 12576-12590. doi: 10.1128/JVI.78.22.12576-12590.2004
[13] ZHOU S, WAN Q J, HUANG Y H, et al. Proteomic analysis of Singapore grouper iridovirus envelope proteins and characterization of a novel envelope protein VP088[J]. Proteomics, 2011, 11(11): 2236-2248. doi: 10.1002/pmic.200900820
[14] HUANG X H, GONG J, HUANG Y H, et al. Characterization of an envelope gene VP19 from Singapore grouper iridovirus[J]. Virol J, 2013, 10: 354. doi: 10.1186/1743-422X-10-354
[15] ZHANG H L, ZHOU S, XIA L Q, et al. Characterization of the VP39 envelope protein from Singapore grouper iridovirus[J]. Can J Microbiol, 2015, 61(12): 924-937. doi: 10.1139/cjm-2015-0118
[16] TENG Y, HOU Z W, GONG J, et al. Whole-genome transcriptional profiles of a novel marine fish iridovirus, Singapore grouper iridovirus (SGIV) in virus-infected grouper spleen cell cultures and in orange-spotted grouper, Epinephuluscoioides[J]. Virology, 2008, 377(1): 39-48. doi: 10.1016/j.virol.2008.04.011
[17] YAN Y, CUI H C, JIANG S, et al. Identification of a novel marine fish virus, Singapore grouper iridovirus-encoded microRNAs expressed in grouper cells by Solexa sequencing[J]. PLoS One, 2011, 6(4): e19148. doi: 10.1371/journal.pone.0019148
[18] XIA L Q, CAO J H, HUANG X H, et al. Characterization of Singapore grouper iridovirus (SGIV) ORF086R, a putative homolog of ICP18 involved in cell growth control and virus replication[J]. Arch Virol, 2009, 154(9): 1409-1416. doi: 10.1007/s00705-009-0457-y
[19] XIA L Q, LIANG H, HUANG Y H, et al. Identification and characterization of Singapore grouper iridovirus (SGIV) ORF162L, an immediate-early gene involved in cell growth control and viral replication[J]. Virus Res, 2010, 147(1): 30-39. doi: 10.1016/j.virusres.2009.09.015
[20] HUANG X H, HUANG Y H, GONG J, et al. Identification and characterization of a putative lipopolysaccharide-induced TNF-alpha factor (LITAF) homolog from Singapore grouper iridovirus[J]. Biochem Bioph Res Co, 2008, 373(1): 140-145. doi: 10.1016/j.bbrc.2008.06.003
[21] CAI J, HUANG Y H, WEI S N, et al. Characterization of LPS-induced TNFα factor (LITAF) from orange-spotted grouper, Epinephelus coioides[J]. Fish Shellfish Immunol, 2013, 35(6): 1858-1866. doi: 10.1016/j.fsi.2013.09.023
[22] HUANG X H, HUANG Y H, CAI J, et al. Identification and characterization of a tumor necrosis factor receptor like protein encoded by Singapore grouper iridovirus[J]. Virus Res, 2013, 178(2): 340-348. doi: 10.1016/j.virusres.2013.09.023
[23] YU Y P, HUANG Y H, WEI S N, et al. A tumour necrosis factor receptor-like protein encoded by Singapore grouper iridovirus modulates cell proliferation, apoptosis and viral replication[J]. J Gen Virol, 2016, 97(3): 756-766. doi: 10.1099/jgv.0.000379
[24] YU Y P, HUANG Y H, NI S W, et al. Singapore grouper iridovirus (SGIV) TNFR homolog VP51 functions as a virulence factor via modulating host inflammation response[J]. Virology, 2017, 511: 280-289. doi: 10.1016/j.virol.2017.06.025
[25] GONG J, HUANG Y H, HUANG X H, et al. Nuclear-export-signal-dependent protein translocation of dUTPase encoded by Singapore grouper iridovirus[J]. Arch Virol, 2010, 155(7): 1069-1076. doi: 10.1007/s00705-010-0684-2
[26] YAN Y, CUI H C, GUO C Y, et al. An insulin-like growth factor homologue of Singapore grouper iridovirus modulates cell proliferation, apoptosis and enhances viral replication[J]. J Gen Virol, 2013, 94: 2759-2770. doi: 10.1099/vir.0.056135-0
[27] YAN Y, CUI H C, GUO C Y, et al. Singapore grouper iridovirus-encoded semaphorin homologue (SGIV-sema) contributes to viral replication, cytoskeleton reorganization and inhibition of cellular immune responses[J]. J Gen Virol, 2014, 95: 1144-1155. doi: 10.1099/vir.0.060608-0
[28] YAN Y, GUO C Y, NI S W, et al. Singapore grouper iridovirus (SGIV) encoded SGIV-miR-13 attenuates viral infection via modulating major capsid protein expression[J]. Virus Res, 2015, 205: 45-53. doi: 10.1016/j.virusres.2015.05.010
[29] GUO C Y, YAN Y, CUI H C, et al. miR-homoHSV of Singapore grouper iridovirus (SGIV) inhibits expression of the SGIV pro-apoptotic factor LITAF and attenuates cell death[J]. PLoS One, 2013, 8(12): e83027. doi: 10.1371/journal.pone.0083027
[30] HUANG C H, ZUANG X, GIN K Y, et al. In situ hybridization of a marine fish virus, Singapore grouper iridovirus with a nucleic acid probe of major capsid protein[J]. J Virol Methods, 2004, 117(2): 123-128. doi: 10.1016/j.jviromet.2004.01.002
[31] FU J, HUANG Y H, CAI J, et al. Identification and characterization of Rab7 from orange-spotted grouper, Epinephelus coioides[J]. Fish Shellfish Immunol, 2014, 36(1): 19-26. doi: 10.1016/j.fsi.2013.10.002
[32] WANG S W, HUANG X H, HUANG Y H, et al. Entry of a novel marine DNA virus, Singapore grouper iridovirus, into host cells occurs via clathrin-mediated endocytosis and macropinocytosis in a pH-dependent manner[J]. J Virol, 2014, 88(22): 13047-13063. doi: 10.1128/JVI.01744-14
[33] PAN Y, WANG S W, SHAN Y, et al. Ultrafast tracking of a single live virion during the invagination of a cell membrane[J]. Small, 2015, 11(23): 2782-2788. doi: 10.1002/smll.201403491
[34] HUANG X H, HUANG Y H, OUYANG Z L, et al. Singapore grouper iridovirus, a large DNA virus, induces nonapoptotic cell death by a cell type dependent fashion and evokes ERK signaling[J]. Apoptosis, 2011, 16(8): 831-845. doi: 10.1007/s10495-011-0616-y
[35] HUANG Y H, HUANG X H, YAN Y, et al. Transcriptome analysis of orange-spotted grouper (Epinephelus coioides) spleen in response to Singapore grouper iridovirus[J]. BMC Genomics, 2011, 12: 556. doi: 10.1186/1471-2164-12-556
[36] HUANG X H, HUANG Y H, OUYANG Z L, et al. Roles of stress-activated protein kinases in the replication of Singapore grouper iridovirus and regulation of the inflammatory responses in grouper cells[J]. J Gen Virol, 2011, 92: 1292-1301. doi: 10.1099/vir.0.029173-0
[37] CAI J, HUANG Y H, WEI S N, et al. Characterization of p38 MAPKs from orange-spotted grouper, Epinephelus coioides involved in SGIV infection[J]. Fish Shellfish Immunol, 2011, 31(6): 1129-1136. doi: 10.1016/j.fsi.2011.10.004
[38] WEI S N, HUANG Y H, HUANG X H, et al. Characterization of c-Jun from orange-spotted grouper, Epinephelus coioides involved in SGIV infection[J]. Fish Shellfish Immunol, 2015, 43(1): 230-240. doi: 10.1016/j.fsi.2014.12.033
[39] GUO M L, WEI J G, ZHOU Y S, et al. MKK7 confers different activities to viral infection of Singapore grouper iridovirus (SGIV) and nervous necrosis virus (NNV) in grouper[J]. Fish Shellfish Immunol, 2016, 57: 419-427. doi: 10.1016/j.fsi.2016.09.002
[40] GUO M L, WEI J G, ZHOU Y S, et al. c-Jun N-terminal kinases 3 (JNK3) from orange-spotted grouper, Epinephelus coioides, inhibiting the replication of Singapore grouper iridovirus (SGIV) and SGIV-induced apoptosis[J]. Dev Comp Immunol, 2016, 65: 169-181. doi: 10.1016/j.dci.2016.06.009
[41] GUO M L, WEI J G, ZHOU Y S, et al. Molecular clone and characterization of c-Jun N-terminal kinases 2 from orange-spotted grouper, Epinephelus coioides[J]. Fish Shellfish Immunol, 2016, 49: 355-363. doi: 10.1016/j.fsi.2015.12.001
[42] GUO M L, WEI J G, HUANG X H, et al. JNK1 derived from orange-spotted grouper, Epinephelus coioides, involving in the evasion and infection of Singapore grouper iridovirus (SGIV)[J]. Front Microbiol, 2016, 7: 121.
[43] BANKS L, PIM D, THOMAS M. Viruses and the 26S proteasome: Hacking into destruction[J]. Trends BiochemSci, 2003, 28(8): 452-459. doi: 10.1016/S0968-0004(03)00141-5
[44] RANDOW F, LEHNER P J. Viral avoidance and exploitation of the ubiquitin system[J]. Nat Cell Biol, 2009, 11(5): 527-534. doi: 10.1038/ncb0509-527
[45] HUANG X H, WEI S N, NI S W, et al. Ubiquitin-proteasome system is required for efficient replication of Singapore grouper iridovirus[J]. Front Microbiol, 2018, 9: 2798. doi: 10.3389/fmicb.2018.02798
[46] HUANG Y H, YU Y P, YANG Y, et al. Fish TRIM8 exerts antiviral roles through regulation of the proinflammatory factors and interferon signaling[J]. Fish Shellfish Immunol, 2016, 54: 435-444. doi: 10.1016/j.fsi.2016.04.138
[47] YANG Y, HUANG Y H, YU Y P, et al. RING domain is essential for the antiviral activity of TRIM25 from orange spotted grouper[J]. Fish Shellfish Immunol, 2016, 55: 304-314. doi: 10.1016/j.fsi.2016.06.005
[48] YU Y P, HUANG X H, LIU J X, et al. Fish TRIM32 functions as a critical antiviral molecule against iridovirus and nodavirus[J]. Fish Shellfish Immunol, 2017, 60: 33-43. doi: 10.1016/j.fsi.2016.11.036
[49] YANG Y, HUANG Y H, YU Y P, et al. Negative regulation of the innate antiviral immune response by TRIM62 from orange spotted grouper[J]. Fish Shellfish Immunol, 2016, 57: 68-78. doi: 10.1016/j.fsi.2016.08.035
[50] LV S Y, ZHAN Y, ZHENG J Y, et al. Negative regulation of the interferon response by finTRIM82 in the orange spotted grouper[J]. Fish Shellfish Immunol, 2019, 88: 391-402. doi: 10.1016/j.fsi.2019.03.004
[51] HUANG Y H, ZHANG J C, LIU J X, et al. Fish TRIM35 negatively regulates the interferon signaling pathway in response to grouper nodavirus infection[J]. Fish Shellfish Immunol, 2017, 69: 142-152. doi: 10.1016/j.fsi.2017.08.019
[52] YU Y P, HUANG X H, ZHANG J C, et al. Fish TRIM16L exerts negative regulation on antiviral immune response against grouper iridoviruses[J]. Fish Shellfish Immunol, 2016, 59: 256-267. doi: 10.1016/j.fsi.2016.10.044
[53] HUANG Y H, YANG M, YU Y P, et al. Grouper TRIM13 exerts negative regulation of antiviral immune response against nodavirus[J]. Fish Shellfish Immunol, 2016, 55: 106-115. doi: 10.1016/j.fsi.2016.05.029
[54] QIN Q W, GIN K Y, LEE L Y, et al. Development of a flow cytometry based method for rapid and sensitive detection of a novel marine fish iridovirus in cell culture[J]. J Virol Methods, 2005, 125(1): 49-54. doi: 10.1016/j.jviromet.2004.12.005
[55] LIU W T, ZHU L, QIN Q W, et al. Microfluidic device as a new platform for immunofluorescent detection of viruses[J]. Lab Chip, 2005, 5(11): 1327-1330. doi: 10.1039/b509086e
[56] MAO X L, ZHOU S, XU D, et al. Rapid and sensitive detection of Singapore grouper iridovirus by loop-mediated isothermal amplification[J]. J ApplMicrobiol, 2008, 105(2): 389-397.
[57] LI P F, YAN Y, WER S N, et al. Isolation and characterization of a new class of DNA aptamers specific binding to Singapore grouper iridovirus (SGIV) with antiviral activities[J]. Virus Res, 2014, 188: 146-154. doi: 10.1016/j.virusres.2014.04.010
[58] LI P F, WEI S N, ZHOU L L, et al. Selection and characterization of novel DNA aptamers specifically recognized by Singapore grouper iridovirus-infected fish cells[J]. J Gen Virol, 2015, 96(11): 3348-3359. doi: 10.1099/jgv.0.000270
[59] LI P F, ZHOU L L, WEI J G, et al. Development and characterization of aptamer-based enzyme-linked apta-sorbent assay for the detection of Singapore grouper iridovirus infection[J]. J Appl Microbiol, 2016, 121(3): 634-643.
[60] ZHANG X B, HUANG C H, QIN Q W. Antiviral properties of hemocyanin isolated from shrimp Penaeus monodon[J]. Antiviral Res, 2004, 61(2): 93-99. doi: 10.1016/j.antiviral.2003.08.019
[61] XU D, WEI J G, CUI H C, et al. Differential profiles of gene expression in grouper Epinepheluscoioides, infected with Singapore grouper iridovirus, revealed by suppression subtractive hybridization and DNA microarray[J]. J Fish Biol, 2010, 77(2): 341-360. doi: 10.1111/jfb.2010.77.issue-2
[62] HUANG Y H, ZHANG J C, OUYANG Z L, et al. Grouper MAVS functions as a crucial antiviral molecule against nervous necrosis virus infection[J]. Fish Shellfish Immunol, 2018, 72: 14-22. doi: 10.1016/j.fsi.2017.10.035
[63] WEI J G, GUO M L, GAO P, et al. Isolation and characterization of tumor necrosis factor receptor-associated factor 6 (TRAF6) from grouper, Epinephelus tauvina[J]. Fish Shellfish Immunol, 2014, 39(1): 61-68. doi: 10.1016/j.fsi.2014.04.022
[64] YU Y P, HUANG Y H, YANG Y, et al. Negative regulation of the antiviral response by grouper LGP2 against fish viruses[J]. Fish Shellfish Immunol, 2016, 56: 358-366. doi: 10.1016/j.fsi.2016.07.015
[65] HUANG Y H, YU Y P, YANG Y, et al. Antiviral function of grouper MDA5 against iridovirus and nodavirus[J]. Fish Shellfish Immunol, 2016, 54: 188-196. doi: 10.1016/j.fsi.2016.04.001
[66] HUANG Y H, OUANG Z L, WANG W, et al. Antiviral role of grouper STING against iridovirus infection[J]. Fish Shellfish Immunol, 2015, 47(1): 157-167. doi: 10.1016/j.fsi.2015.09.014
[67] HU Y, HUANG Y H, LIU J X, et al. TBK1 from orange-spotted grouper exerts antiviral activity against fish viruses and regulates interferon response[J]. Fish Shellfish Immunol, 2018, 73: 92-99. doi: 10.1016/j.fsi.2017.12.010
[68] HUANG Y H, HUANG X H, CAI J, et al. Identification of orange-spotted grouper (Epinephelus coioides) interferon regulatory factor 3 involved in antiviral immune response against fish RNA virus[J]. Fish Shellfish Immunol, 2015, 42(2): 345-352. doi: 10.1016/j.fsi.2014.11.025
[69] CUI H C, YAN Y, WEI J G, et al. Identification and functional characterization of an interferon regulatory factor 7-like (IRF7-like) gene from orange-spotted grouper, Epinephelus coioides[J]. Dev Comp Immunol, 2011, 35(6): 672-684. doi: 10.1016/j.dci.2011.01.021
[70] GAO R, HUANG Y H, HUANG X H, et al. Molecular cloning and characterization of two types of IκBα orthologues in orange-spotted grouper, Epinephelus coioides[J]. Fish Shellfish Immunol, 2014, 38(1): 101-110. doi: 10.1016/j.fsi.2014.02.019
[71] HUANG Y H, HUANG X H, YANG Y, et al. Involvement of fish signal transducer and activator of transcription 3 (STAT3) in nodavirus infection induced cell death[J]. Fish Shellfish Immunol, 2015, 43(1): 241-248. doi: 10.1016/j.fsi.2014.12.031
[72] ZHANG J C, HUANG X H, NI S W, et al. Grouper STAT1a is involved in antiviral immune response against iridovirus and nodavirus infection[J]. Fish Shellfish Immunol, 2017, 70: 351-360. doi: 10.1016/j.fsi.2017.09.030
[73] WEI J G, XU D, ZHOU J G, et al. Molecular cloning, characterization and expression analysis of a C-type lectin (Ec-CTL) in orange-spotted grouper, Epinephelus coioides[J]. Fish Shellfish Immunol, 2010, 28(1): 178-186. doi: 10.1016/j.fsi.2009.10.020
[74] JI H S, WEI J G, WEI S N, et al. Molecular cloning and expression of a C-type lectin-like protein from orange-spotted grouper Epinephelus coioides[J]. J Fish Biol, 2014, 84(2): 436-447. doi: 10.1111/jfb.2014.84.issue-2
[75] CHEN X L, WEI J G, XU M, et al. Molecular cloning and characterization of a galectin-1 homolog in orange-spotted grouper, Epinephelus coioides[J]. Fish Shellfish Immunol, 2016, 54: 333-341. doi: 10.1016/j.fsi.2016.02.036
[76] ZHOU JG, WEI JG, XU D, et al. Molecular cloning and characterization of two novel hepcidins from orange-spotted grouper, Epinephelus coioides[J]. Fish Shellfish Immunol, 2011, 30(2): 559-568. doi: 10.1016/j.fsi.2010.11.021
[77] GUO M L, WEI J G, HUANG X H, et al. Antiviral effects of β-defensin derived from orange-spotted grouper (Epinephelus coioides)[J]. Fish Shellfish Immunol, 2012, 32(5): 828-838. doi: 10.1016/j.fsi.2012.02.005
[78] WEI S N, HUANG Y H, CAI J, et al. Molecular cloning and characterization of c-type lysozyme gene in orange-spotted grouper, Epinephelus coioides[J]. Fish Shellfish Immunol, 2012, 33(2): 186-196. doi: 10.1016/j.fsi.2012.03.027
[79] WEI S N, HUANG Y H, HUANG X H, et al. Molecular cloning and characterization of a new G-type lysozyme gene (Ec-lysG) in orange-spotted grouper, Epinephelus coioides[J]. Dev Comp Immunol, 2014, 46(2): 401-412. doi: 10.1016/j.dci.2014.05.006
[80] OUYANG Z L, WANG P R, HUANG X H, et al. Immunogenicity and protective effects of inactivated Singapore grouper iridovirus (SGIV) vaccines in orange-spotted grouper, Epinephelus coioides[J]. Dev Comp Immunol, 2012, 38(2): 254-261. doi: 10.1016/j.dci.2012.07.004
[81] OUYANG Z L, WANG P R, HUANGY H, et al. Selection and identification of Singapore grouper iridovirus vaccine candidate antigens using bioinformatics and DNA vaccination[J]. Vet Immunol Immunopathol, 2012, 149(1/2): 38-45.



 下载:
下载: