Study on hydrolysis kinetics of azadirachtin and tructure analysis of hydrolysate
-
摘要:目的
系统研究印楝素在水溶液中的水解。
方法硅胶柱层析法和半制备液相色谱法分离纯化w为44.56%的印楝素原药中的印楝素A,采用核磁共振仪和高效液相色谱定性、定量测定分离得到的印楝素A,建立一种检测水样中印楝素残留的高效液相色谱方法。
结果核磁共振仪和高效液相色谱测得印楝素A的质量分数分别为90.37%和91.82%。当印楝素添加水平为0.1、1.0和5.0 mg·kg-1时,水样中印楝素的平均回收率为92.53%~94.12%,变异系数为0.35%~0.84%,最小检测质量浓度为0.012 mg·L-1。印楝素在pH 4.0~6.0的缓冲溶液中稳定,当pH大于8.0时,印楝素降解加快,降解半衰期从pH 8.0的14.856 h降到pH 10.0的0.033 h。在pH 6.0的缓冲溶液中,25、35、45 ℃条件下印楝素的降解半衰期分别为24.68、13.69和2.36 d,而在pH 7.0的缓冲溶液中印楝素的降解半衰期分别为9.35、6.51和0.94 d。在pH 2.0的缓冲溶液中分离纯化水解产物得到印楝素A内酯衍生物。
结论印楝素在碱性环境下极不稳定,而在弱酸性环境中比较稳定。温度对印楝素的降解影响很大,随着温度的升高印楝素降解加快。
Abstract:ObjectiveTo study hydrolysis of azadirachtin in water systematically.
MethodAzadirachtin A from the 44.56% azadirachtin TC was isolated and purified by silica column chromatography and semi-preparative high performance liquid chromatography (HPLC). The chemical structure and content of isolated azadirachtin A were identified by nulear magnetic resonance (NMR) and HPLC. A method for determining azaditachtin residue in water by HPLC was established.
ResultThe mass fractions of azadirachtin A were 90.37% and 91.82% detected by NMR and HPLC respectively. When azadirachtin was added with the concentrations of 0.1, 1.0 and 5.0 mg·kg-1, the average recovery rates of azadirachtin from water samples ranged from 92.53% to 94.12%, the variation coefficients ranged from 0.35% to 0.84%, and the minimum detection limit was 0.012 mg·L-1. Azadirachtin was stable in buffer solutions with pH varying from 4.0 to 6.0. When pH was above 8.0, hydrolysis of azadirachtin was accelerated, and the degradation half-life was 14.856 h at pH 8.0 and declined to 0.033 h at pH 10.0. The degration half-lives of azadirachtin in buffer solutions at pH 6.0 were 24.68, 13.69 and 2.36 d under 25, 35 and 45 ℃ temperature respectively, while were 9.35, 6.51 and 0.94 d at pH 7.0. A lactone derivative of azadirachtin was obtained by isolating and purifing hydrolysate in buffer solution at pH 2.0.
ConclusionAzadirachtin is extremely unstable in alkaline environment while relatively stable in weak acid environment. Temperature has a great effect on the degradation of azadirachtin and the degradation accelerates as temperature increases.
-
Keywords:
- azadirachtin /
- residue /
- hydrolysis kinetics /
- hydrolysate /
- silica column chromatography /
- HPLC /
- nuclear magnetic resonance
-
印楝Azadirachta indica植株中发现的以印楝素为主的400余种杀虫活性物质,可防治10目400余种农林、仓库和卫生害虫[1-3]。农药的水解是农药的一个主要环境化学行为,包括农药在水环境中的微生物降解、化学降解和光降解,它是评价农药在水体中残留特性的重要指标,其降解速率受农药的性质与水环境条件等制约,而水解只是影响农药含量的一个因素,其他因素如农药的施用量、稀释程度、吸附、生物富集等也影响农药在水环境中的存在状况[4-5]。影响农药水解的因素很多,如反应介质溶剂化能力的变化将影响农药、中间体或产物的水解反应;离子强度和有机溶剂量的改变将影响到溶剂化的能力[6],并且因此改变水解速率。此外还可能存在普通酸、碱和沉积物及痕量金属催化的特殊介质效应[7-9]。有研究从水分含量、能量和温度的角度进行了农药降解试验[10-12]。印楝素的稳定性是田间使用时面临的首要问题[13]。关于印楝素的检测,已有许多相关研究报道[14-17],本文研究pH、温度以及不同水质对印楝素水解的影响,拟建立一种检测水样中印楝素残留的高效液相色谱方法,并对印楝素在pH 2.0的缓冲溶液中的水解产物进行分离、纯化、鉴定,以期为印楝素的科学使用提供理论依据。
1. 材料与方法
1.1 材料
试验所用珠江水采集于珠江中山大学江段;稻田水采集于华南农业大学试验田;地表水采集于火炉山森林公园;水库水采集于龙洞霄鸡坳水库;湖水采集于华南农业大学校内人工湖;纯水为实验室自制。试验所用缓冲溶液配方参考文献[18]:1) pH 1.0:0.2 mol·L-1的HCl 47.5 mL+0.2 mol·L-1的KCl 25 mL;2) pH 2.0:0.2 mol·L-1的HCl 5.3 mL+0.2 mol·L-1的KCl 25 mL;3) pH 3.0:0.2 mol·L-1的HCl 10.2 mL+0.2 mol·L-1的KHC8H4O4 25 mL;4) pH 4.0:0.1 mol·L-1的NaOH 0.4 mL+0.2 mol·L-1的KHC8H4O4 25 mL;5) pH 5.0:0.1 mol·L-1的NaOH 23.9 mL+0.2 mol·L-1的KCl 25 mL;6) pH 6.0:0.1 mol·L-1的NaOH 5.7 mL+0.2 mol·L-1的KH2PO4 25 mL;7) pH 7.0:0.1 mol·L-1的NaOH 29.6 mL+0.2 mol·L-1的KH2PO4 25 mL;8) pH 8.0:0.1 mol·L-1 NaOH 46.8 mL+0.2 mol·L-1 KH2PO4 25 mL;9) pH 9.0:0.1 mol·L-1 NaOH 21.3 mL+0.2 mol·L-1(H3BO3+KCl)25 mL;10) pH 10.0:0.1 mol·L-1的NaOH 43.9 mL+0.2 mol·L-1的(H3BO3+KCl)25 mL;11) pH 11.0:0.1 mol·L-1的NaOH 5.1 mL+0.2 mol·L-1的Na2HPO4 25 mL;12) pH 12.0:0.1 mol·L-1的NaOH 26.9 mL+0.2 mol·L-1的Na2HPO4 25 mL;13) pH 13.0:0.2 mol·L-1的NaOH 66 mL+0.2 mol·L-1的KCl 25 mL, 以上溶液均加蒸馏水定容至100 mL。
1.2 方法
1.2.1 水样理化性质检测
玻璃电极法测定pH、紫外分光光度法测定总氮含量、钼酸铵分光光度法测定总磷含量、重铬酸盐法测定化学需氧量、酚二磺酸分光光度法测定硝酸盐氮含量、纳氏试剂比色法测定氨氮含量。
1.2.2 印楝素及印楝素降解产物柱层析分离
确定洗脱系统:根据待分离物质在GF254板上的分离效果确定洗脱系统和梯度。装柱:将起始洗脱剂溶液V(石油醚):V(乙酸乙酯)=3:7加入硅胶,充分搅拌以排出气泡,一次性加入固定好的层析柱中,打开下部活塞,使溶剂缓缓流出,同时轻轻敲打柱体(注意保持柱面平整)以排出气泡,将流出的洗脱剂加回柱顶部反复冲洗柱中硅胶,直到硅胶面不再下降为止。柱层析硅胶与分离样品的质量比为15~20。拌样:样品溶于少量的丙酮溶剂中,滴入硅胶中(200~300目), 边滴边搅拌,等丙酮完全挥发后,用研磨棒将样品碾匀。上样:将拌好样品的硅胶通过漏斗加入到层析柱的上端,用少量石油醚清洗研钵、漏斗和柱壁上的样品,等样品沉降形成均匀的薄层后,再加入约1 cm的无水硫酸钠或石英砂,然后加入流动相洗脱。洗脱:分别选用正己烷、乙酸乙酯不同体积配比的混合液作为洗脱液。定量接取馏分并蒸干。TLC点样:收集的不同样品用毛细管吸取少量的样品溶液,点于薄层层析板底端约1cm处,各样点成一条直线,与板底线平行。展开剂的高度低于样品线。TLC展开剂为V(乙酸乙酯):V(正己烷)=2:1,随配随用,乙酸乙酯与正己烷的比例随展开效果的不同而变化。待溶剂线展至层析板顶端未至硅胶边缘时,取出层析板,自然晾干,放入充满碘蒸气的碘缸中显色。对收集的组分进行TLC检测,将只出现1个点的组分根据比移值(Rf)是否相同进行合并。
1.2.3 印楝素水解产物的分离鉴定
准确称取0.2 g印楝素溶于100 mL pH为2.0的缓冲液中(加少量甲醇以提高印楝素的溶解度)。然后将该反应液置于70 ℃的水浴锅中,于4 h后进行萃取、浓缩,硅胶柱层析分离。
1.2.4 印楝素及印楝素降解产物的结构鉴定
核磁共振氢谱(1H NMR)、核磁共振碳谱(13C NMR)用Bruker Avance-600型超导核磁共振仪测定,以氘代三氯甲烷(CDCl3)为溶剂,以四甲基硅烷(TMS)为内标。
1.2.5 印楝素残留分析前样品处理
取50 mL已过滤水样(pH 6.8) 于250 mL分液漏斗中,用二氯甲烷萃取3次,用量分别为30、20和20 mL,静置分层,萃取液经无水硫酸钠脱水滤入250 mL的圆底烧瓶中,在40 ℃条件下减压浓缩到1 mL左右,用甲醇定容到3 mL,待测。水样中印楝素残留直接采用二氯甲烷进行萃取[19]。
1.2.6 色谱检测条件
定量检测采用外标法。色谱柱为Agilent TC-C18(5 μm),250 mm×4.6 mm,流动相为V(乙腈): V(水)=40:60,流速为1 mL·min-1,紫外检测波长为217 nm,进样量为10 μL。在上述检测条件下印楝素的保留时间为9.80 min。
1.2.7 不同pH条件下的水解
取洁净且已灭菌的20 mL具塞刻度试管,分为13组,每组30个。准确移取0.5 mg·mL-1的印楝素丙酮溶液2 mL于具塞刻度试管中,待溶剂挥发近干时,分别加入适量的pH为1.0~13.0的缓冲液至20 mL刻度处,振荡使之混和均匀,使印楝素的初始质量浓度为50 mg·L-1,密封后置于(25±1) ℃恒温箱中降解(黑暗避光),每处理3个重复。
在加药当日(0 d)和加药后1、3、5、7、9、15、20、25、30 d取样测定pH为2.0~7.0的缓冲溶液中印楝素的残留量;而pH 1.0和pH为8.0~13.0的缓冲溶液在加药后0、2、4、6、8、10 h取样测定印楝素的残留量。
1.2.8 不同温度条件下的水解
取洁净且已灭菌的具塞刻度试管,分为6组,每组24个。准确移取0.5 mg·mL-1的印楝素丙酮溶液2 mL于具塞刻度试管中,待溶剂挥发近干时,分别加入适量的pH 7.0的缓冲溶液至20 mL刻度处,振荡使之混和均匀,使印楝素的初始质量浓度为50 mg·L-1,密封后分别置于(25±1)、(35±1)、(45±1) ℃的恒温环境中降解(黑暗避光),每处理3个重复。在加药当日(0 d后)和加药后1、3、5、7、9、15、20、25、30 d取样测定印楝素的残留量。
1.2.9 在实际水样中的水解
取洁净且已灭菌的具塞刻度试管,分为5组,每组30个。准确移取0.5 mg·mL-1的印楝素丙酮溶液2 mL于具塞刻度试管中,待溶剂挥发近干时分别加入适量的pH为1.0~10.0的缓冲液以及5种环境天然水样作为试验对象,使样品中印楝素的初始质量分别为0.5、1.0、5.0 mg·L-1,加入适量的NaCl,用二氯甲烷萃取、回收,计算添加回收率。相同处理后使印楝素的初始质量浓度为50 mg·L-1,密封后置于(25±1) ℃恒温箱中降解(黑暗避光),每处理3个重复。在加药当日(0 d)和加药后1、3、5、7、9、15、20、25、30 d取样测定印楝素的残留量。
将印楝素在5种天然水体中的降解半衰期与各水体性质如pH、总氮含量、总磷含量、化学需氧量、硝酸盐氮含量和氨氮含量进行单因子线性回归分析,分析影响印楝素降解的显著理化因素。
1.3 数据处理
根据所测峰面积,计算样本中农药的残留量(R),公式如下:
$$ R=\frac{{{S}_{2}}\times \rho \times {{V}_{1}}\times V}{{{S}_{1}}\times {{V}_{2}}\times m}, $$ 式中,ρ为标准溶液质量浓度,mg·L-1;S1为注入标准溶液的峰面积,S2为注入样品溶液的峰面积,V为样品溶液最终定容体积,mL;V1为标准溶液进样体积,μL;V2为样品溶液进样体积,μL;m为称样质量,g。
试验数据采用DPS软件分析,麦夸特法将水解试验数据拟合一级动力学方程得光解速率常数(k)。
2. 结果与分析
2.1 印楝素的分离纯化及结构鉴定
2.1.1 硅胶柱层析分离纯化
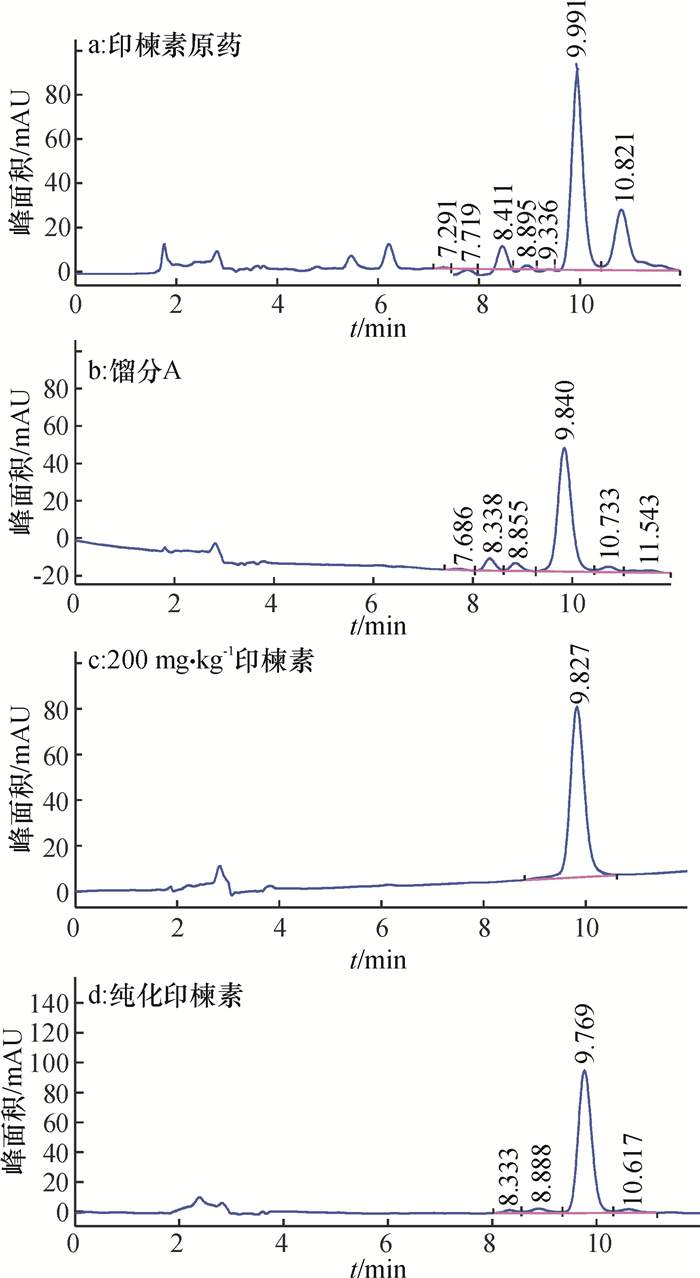
对w为44.56%的印楝素原药进行大柱初步分离并经TLC鉴定,对比印楝素原药、印楝素原药中纯度较高的馏分A和印楝素标准品HPLC法得到的色谱图(图 1a~1c),根据保留时间,发现该馏分A(图 1b)与印楝素标准品谱图吻合,进而对馏分再经硅胶柱层析,以石油醚+乙酸乙酯洗脱,经HPLC分析(图 1d),与标准谱图 1c比较,分析、计算得到印楝素的质量分数为90.37%。
2.1.2 印楝素结构鉴定
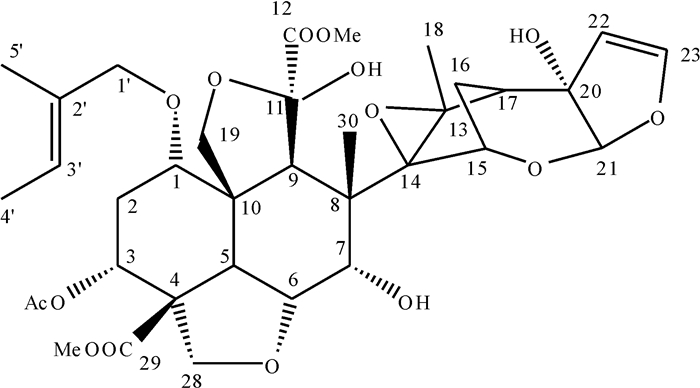
经1H NMR和13C NMR分析,其值与文献基本一致[20],证实该晶体为印楝素,其核磁共振谱的化学位移13C NMR与文献值的比较见表 1,印楝素结构式见图 2。
表 1 印楝素的核磁共振碳谱数据13C NMRTable 1. 13C NMR data of azadirachtin ×10-6碳原子
编号化学位移 文献值[20] 实测值 C-1 70.7 70.6 C-2 29.8 29.7 C-3 67.1 67.0 C-4 45.6 45.6 C-5 37.1 36.9 C-6 74.5 74.3 C-7 76.5 76.5 C-8 50.4 50.3 C-9 44.9 44.7 C-10 52.6 52.5 C-11 104.2 104.2 C-12 169.5 169.8 C-13 70.1 70.1 C-14 68.6 68.8 C-15 73.9 74.0 C-16 25.1 25.0 C-17 48.7 48.7 C-18 20.8 21.0 C-19 69.1 69.0 C-20 83.6 83.4 C-21 107.5 107.6 C-22 108.8 108.7 C-23 147.0 146.8 C-28 73.0 72.9 C-29 166.2 166.3 C-30 18.4 18.5 3-C=O 173.3 173.5 CH3 21.4 21.4 29-OCH3 52.7 52.8 12-OCH3 53.1 53.2 O-Tig; C-1′ 171.8 171.9 C-2′ 128.8 128.6 C-3′ 137.5 137.9 C-4′ 14.2 14.3 C-5′ 11.9 11.9 2.2 降解产物的分离鉴定
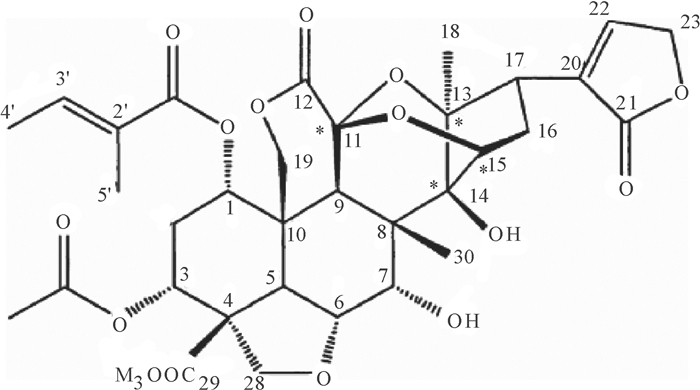
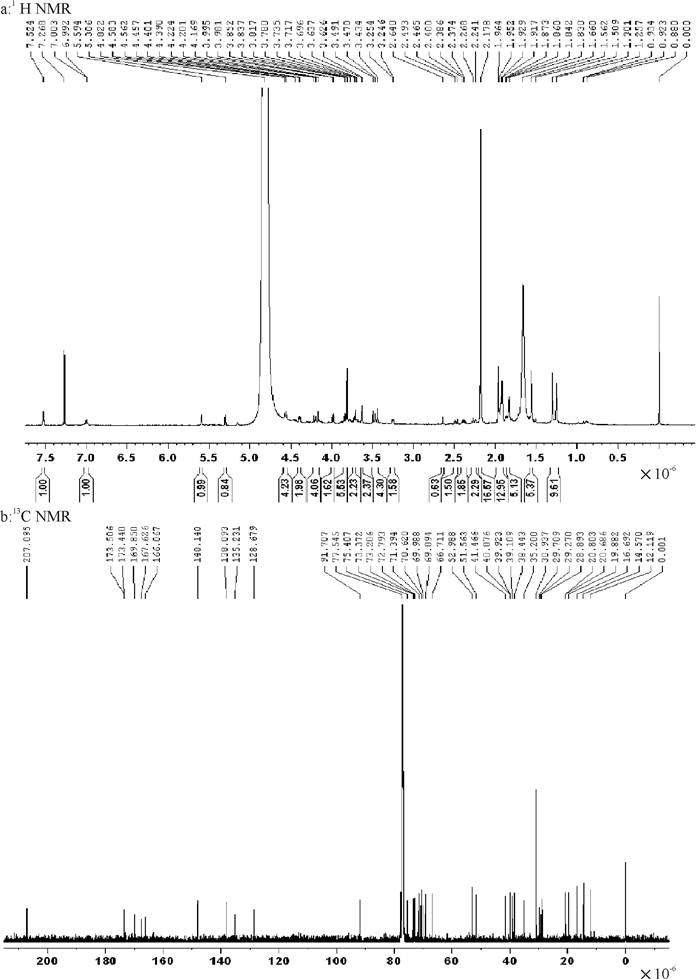
通过研究印楝素在纯净水中水解过程的液相色谱图,发现印楝素在水解后出现了多个水解产物峰,印楝素水解产物经萃取、浓缩后,样品经硅胶柱层析分离纯化, 得到一种纯度较高的印楝素A内脂衍生物, 经1H NMR和13C NMR分析,其结构见图 3,其1H NMR谱见图 4a,13C NMR谱见图 4b。
2.3 印楝素的水化学降解
2.3.1 印楝素在水体中的添加回收试验
pH为1.0和pH为8.0~10.0的缓冲溶液中的添加回收率比较低,少于80%,因为印楝素在碱性和偏酸性的条件下不稳定,容易降解,而pH大于11.0时,难以回收到印楝素。其他情况下添加回收率为82.49%~94.31%,变异系数最大为2.88%,符合残留检测条件。
2.3.2 印楝素的降解试验
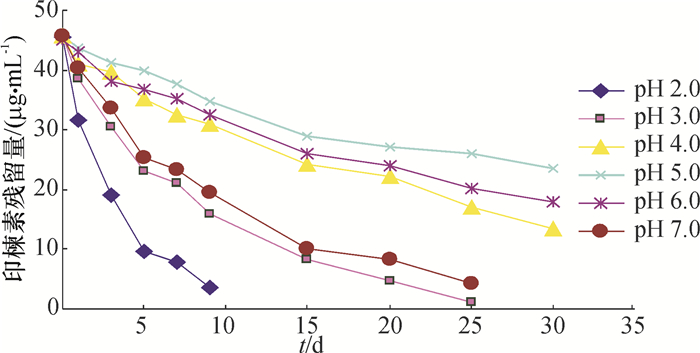
印楝素在pH 2.0~7.0的缓冲溶液中的降解趋势见图 5,采用麦夸特法将试验数据用动力学方程拟合,结果见表 2。可以看出印楝素在pH 4.0、5.0、6.0的缓冲溶液中比较稳定,半衰期分别为22.87、36.33和24.68 d。
表 2 印楝素的动力学分析Table 2. Kinetic analysis of azadirachtinpH 动力学方程 相关系数(r) 半衰期/d 1.0 Ct=44.31e -0.300 0t 0.999 8 0.10 2.0 Ct=42.57e-0.233 0t 0.997 6 2.98 3.0 Ct=44.61e-0.079 8t 0.997 4 8.68 4.0 Ct=44.03e-0.030 3t 0.994 8 22.87 5.0 Ct=44.85e-0.019 1t 0.991 2 36.33 6.0 Ct=44.36e-0.028 1t 0.997 6 24.68 7.0 Ct=43.04e-0.074 1t 0.997 7 9.35 8.0 Ct=43.73e-0.026 7t 0.975 4 0.62 9.0 Ct=45.16e-0.289 0t 0.998 8 0.10 10.0 Ct=44.99e-0.877 0t 0.999 9 0.03 印楝素在pH 8.0~10.0和pH 1.0的缓冲溶液中的降解趋势见图 6,采用麦夸特法将试验数据用动力学方程拟合(表 2)。从图 6和表 2可以看出印楝素在pH 1.0和碱性条件下降解很快,在pH为1.0、8.0、9.0和10.0缓冲溶液中降解的半衰期分别为0.10、0.62、0.10和0.03 d,而在pH大于11.0的缓冲液中的半衰期小于10 min。
由表 2可以看出,印楝素在pH 4.0~6.0的缓冲溶液中比较稳定,而当pH大于8.0时,印楝素降解速率显著加快,降解半衰期从pH 8.0的0.62 d降到pH 10.0的0.03 d,而pH大于11.0时则难以检测到印楝素的残留,印楝素在pH小于3.0的缓冲溶液中的半衰期下降的趋势小于pH大于8.0的下降趋势。总之,印楝素在碱性环境下不稳定,而在弱酸性环境中比较稳定。
2.3.3 温度对印楝素水解速率的影响
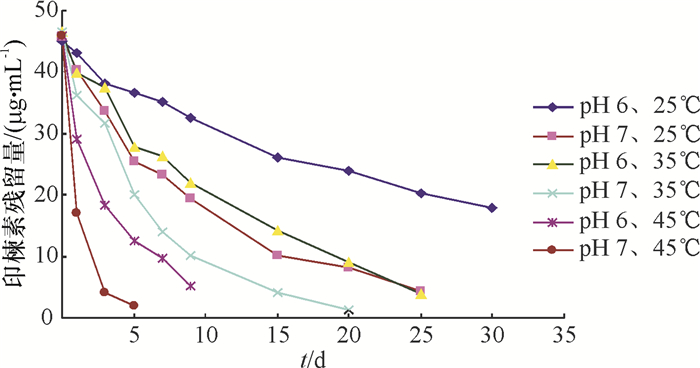
印楝素在模拟天然水体中不同温度下的降解趋势见图 7,采用麦夸特法将试验数据用动力学方程拟合,结果见表 3。从表 3可以看出,温度对印楝素在模拟天然水体中的降解影响很大,在pH 6.0的缓冲溶液中,25、35和45 ℃下的降解半衰期分别为24.68、13.69和2.36 d,而在pH 7.0的缓冲溶液中分别为9.35、6.51和0.94 d,可见,当温度高于35 ℃时,印楝素的降解速率明显加快,也可以看出,水体的pH对印楝素的降解也有很大的影响。
表 3 印楝素在模拟天然水体不同温度下的降解动力学方程Table 3. Degradation kinetic equations of azadirachtin in simulated natural water at different temperaturespH θ/℃ 动力学方程 相关系数(r) 半衰期/d 6.0 25 Ct=44.36e-0.028 1t 0.997 6 24.68 35 Ct=45.08e-0.050 6t 0.994 3 13.69 45 Ct=47.63e-0.294 0t 0.992 4 2.36 7.0 25 Ct=43.04e-0.074 1t 0.997 7 9.35 35 Ct=45.57e-0.106 0t 0.994 5 6.51 45 Ct=45.98e-0.737 0t 0.999 9 0.94 2.3.4 印楝素在天然水体中的水解动力学
印楝素在天然水体中的降解结果见表 4。从表 4可以看出,印楝素在水库水中的降解半衰期最长,为37.06 d,在湖水中的半衰期最短,为6.43 d,在地表水和稻田水中降解的半衰期相差不大,分别为15.90和14.91 d,在珠江水中的降解半衰期相对较小,为12.19 d。将印楝素在水体中降解的半衰期与水体理化性质进行单因子线性回归分析(表 5),从表 5可以看出印楝素在水体中的降解与pH表现出良好的相关性,相关系数为0.995 9,而与总氮含量、总磷含量、化学需氧量、硝酸盐氮含量和氨氮含量的相关性很差。
表 4 印楝素在天然水体中的降解动力学方程Table 4. Degradation kinetic equations of azadirachtin in natural water水体 动力学方程 相关系数(r) 半衰期/d 珠江水 Ct=46.76e-0.056 9t 0.997 4 12.19 稻田水 Ct=45.98e-0.046 5t 0.989 7 14.91 地表水 Ct=45.72e-0.043 6t 0.996 6 15.90 湖水 Ct=44.79e-0.108 0t 0.994 2 6.43 水库水 Ct=44.96e-0.018 0t 0.995 0 37.06 表 5 印楝素降解半衰期与水体理化性质的相关性分析Table 5. Analysis of the correlation between the degradation half-life of azadirachtin and physical/chemical properties of water理化性质 回归方程1) 相关系数(r) pH y=183.570-23.537 0x 0.995 9 总磷含量 y=20.995-11.123 0x 0.285 5 总氮含量 y=27.190-2.367 8x 0.498 0 化学需氧量 y=18.449-0.009 3x 0.044 7 硝酸盐氮含量 y=20.352-2.726 1x 0.376 8 氨氮含量 y=16.873+0.890 6x 0.319 1 1)y为印楝素半衰期,x为对应的理化性质。 3. 结论
印楝素在不同温度下的水解试验表明,温度对其降解影响较大,在pH 6.0的缓冲溶液中,25、35和45 ℃下的降解半衰期分别为24.68、13.69和2.36 d,而在pH 7.0的缓冲溶液中分别为9.35、6.51、0.94 d,可见,当温度高于35 ℃时,印楝素A的降解速率明显加快,所以在印楝素的储存过程中,要尽量避免高温。在研究印楝素水解产物时发现,HPLC色谱图上显示有多个水解产物峰,但只分离到1种纯度较高的产物,通过硅胶柱层析对印楝素的水解产物进行了分离及结构鉴定,确定分离的水解产物为印楝素A内酯衍生物。其他的水解产物有待进一步的分离和结构鉴定。本研究通过硅胶柱层析对w为44.56%的印楝素原药进行分离纯化,可以得到w为90%以上的印楝素原药。水解试验表明,印楝素在弱酸性环境中比较稳定,而碱性环境下极不稳定。印楝素在天然水体中的降解受pH影响显著,而与总氮含量、总磷含量、化学需氧量、硝酸盐氮和氨氮含量等因子的相关性不明显。
印楝素水解产物的结构表征、合成及其在环境中的归宿与生态毒理效应还有待进一步的研究。
-
表 1 印楝素的核磁共振碳谱数据13C NMR
Table 1 13C NMR data of azadirachtin ×10-6
碳原子
编号化学位移 文献值[20] 实测值 C-1 70.7 70.6 C-2 29.8 29.7 C-3 67.1 67.0 C-4 45.6 45.6 C-5 37.1 36.9 C-6 74.5 74.3 C-7 76.5 76.5 C-8 50.4 50.3 C-9 44.9 44.7 C-10 52.6 52.5 C-11 104.2 104.2 C-12 169.5 169.8 C-13 70.1 70.1 C-14 68.6 68.8 C-15 73.9 74.0 C-16 25.1 25.0 C-17 48.7 48.7 C-18 20.8 21.0 C-19 69.1 69.0 C-20 83.6 83.4 C-21 107.5 107.6 C-22 108.8 108.7 C-23 147.0 146.8 C-28 73.0 72.9 C-29 166.2 166.3 C-30 18.4 18.5 3-C=O 173.3 173.5 CH3 21.4 21.4 29-OCH3 52.7 52.8 12-OCH3 53.1 53.2 O-Tig; C-1′ 171.8 171.9 C-2′ 128.8 128.6 C-3′ 137.5 137.9 C-4′ 14.2 14.3 C-5′ 11.9 11.9 表 2 印楝素的动力学分析
Table 2 Kinetic analysis of azadirachtin
pH 动力学方程 相关系数(r) 半衰期/d 1.0 Ct=44.31e -0.300 0t 0.999 8 0.10 2.0 Ct=42.57e-0.233 0t 0.997 6 2.98 3.0 Ct=44.61e-0.079 8t 0.997 4 8.68 4.0 Ct=44.03e-0.030 3t 0.994 8 22.87 5.0 Ct=44.85e-0.019 1t 0.991 2 36.33 6.0 Ct=44.36e-0.028 1t 0.997 6 24.68 7.0 Ct=43.04e-0.074 1t 0.997 7 9.35 8.0 Ct=43.73e-0.026 7t 0.975 4 0.62 9.0 Ct=45.16e-0.289 0t 0.998 8 0.10 10.0 Ct=44.99e-0.877 0t 0.999 9 0.03 表 3 印楝素在模拟天然水体不同温度下的降解动力学方程
Table 3 Degradation kinetic equations of azadirachtin in simulated natural water at different temperatures
pH θ/℃ 动力学方程 相关系数(r) 半衰期/d 6.0 25 Ct=44.36e-0.028 1t 0.997 6 24.68 35 Ct=45.08e-0.050 6t 0.994 3 13.69 45 Ct=47.63e-0.294 0t 0.992 4 2.36 7.0 25 Ct=43.04e-0.074 1t 0.997 7 9.35 35 Ct=45.57e-0.106 0t 0.994 5 6.51 45 Ct=45.98e-0.737 0t 0.999 9 0.94 表 4 印楝素在天然水体中的降解动力学方程
Table 4 Degradation kinetic equations of azadirachtin in natural water
水体 动力学方程 相关系数(r) 半衰期/d 珠江水 Ct=46.76e-0.056 9t 0.997 4 12.19 稻田水 Ct=45.98e-0.046 5t 0.989 7 14.91 地表水 Ct=45.72e-0.043 6t 0.996 6 15.90 湖水 Ct=44.79e-0.108 0t 0.994 2 6.43 水库水 Ct=44.96e-0.018 0t 0.995 0 37.06 表 5 印楝素降解半衰期与水体理化性质的相关性分析
Table 5 Analysis of the correlation between the degradation half-life of azadirachtin and physical/chemical properties of water
理化性质 回归方程1) 相关系数(r) pH y=183.570-23.537 0x 0.995 9 总磷含量 y=20.995-11.123 0x 0.285 5 总氮含量 y=27.190-2.367 8x 0.498 0 化学需氧量 y=18.449-0.009 3x 0.044 7 硝酸盐氮含量 y=20.352-2.726 1x 0.376 8 氨氮含量 y=16.873+0.890 6x 0.319 1 1)y为印楝素半衰期,x为对应的理化性质。 -
[1] 荣晓东, 徐汉虹, 赵善欢.植物性杀虫剂印楝的研究进展[J].农药学学报, 2000(2): 9-14. http://www.cnki.com.cn/Article/CJFDTOTAL-NYXB200002001.htm [2] 谭卫红, 宋湛谦.天然植物杀虫剂印楝素的研究进展[J].华南热带农业大学学报, 2004, 10(1): 23-29. http://www.cnki.com.cn/Article/CJFDTOTAL-HEBY200505017.htm [3] 赵淑英, 王秋, 罗万春.印楝植物农药的研究进展[J].济南大学学报, 2004, 18(2): 145-149. http://www.cnki.com.cn/Article/CJFDTOTAL-SDJC200402015.htm [4] 张宗炳, 樊德方, 钱传范, 等.杀虫药剂环境毒理学[M].北京:中国农业出版社, 1989: 25-89. [5] 蔡道基.农药环境毒理学研究[M].北京:中国环境科学出版社, 1999: 78-83. [6] WEI J, FURRER G, SCHULIN R. Kinetics of carbosulfan degradation in the aqueous phase in the presence of a cosolvent[J]. J Environ Qual, 2000, 29(5): 1481-1487. https://www.cabdirect.org/cabdirect/abstract/20003010362
[7] 王连生.有机污染化学[M].北京:科学出版社, 1990: 208-255. [8] SARMAH A K, KOOKANA R S, DUFFY M J, et al. Hydrolysis of triasulfuron, metsulfuron-methyl and chlorsulfuron in alkaline soil and aqueous solutions[J]. Pest Manag Sci, 2000, 56(5): 463-471. doi: 10.1002/(ISSN)1526-4998
[9] WET J, FURRIE G, KAUFMANN S, et al. Influence of clay minerals on the hydrolysis of carbamate pesticides[J]. Environ Sci Technol, 2001, 35(11): 2226-2232. doi: 10.1021/es000179d
[10] 韩丙军, 何书海, 林靖凌, 等.温度影响印楝素A及同系物降解动力学研究[J].现代农药, 2007(6): 30-34. http://www.cnki.com.cn/Article/CJFDTOTAL-NYXD200706012.htm [11] 韩丙军, 陈丽霞, 黄华平, 等.印楝素A降解动力学研究[J].热带作物学报, 2008(1): 97-101. http://www.cnki.com.cn/Article/CJFDTOTAL-RDZX200801023.htm [12] 严亚丽, 陈丽霞, 彭黎旭.水分影响印楝素及同系物降解动力学研究[J].热带农业科学, 2009(5): 30-32. http://www.cnki.com.cn/Article/CJFDTOTAL-RDNK200905007.htm [13] 徐勇, 郭鑫宇, 项盛, 等.植物源杀虫剂印楝素研究开发及应用进展[J].现代农药, 2014(5): 31-37. http://www.cnki.com.cn/Article/CJFDTOTAL-NYXD201405010.htm [14] 罗应兰, 刘宏程.超声波固相萃取-高效液相色谱法同时测定茶叶中印楝素及类似物残留量[J].农药, 2016 (12): 915-917. http://www.cnki.com.cn/Article/CJFDTOTAL-NYZZ201612020.htm [15] 周颖, 安莹, 燕传勇, 等.超高效液相色谱-串联质谱法测定茶叶中印楝素A和B的残留量[J].理化检验, 2016, 52(11): 1292-1296. doi: 10.11973/lhjy-hx201611011 [16] GAI M N, ÁLVAREZ C, VENEGAS R, et al. An HPLC method for determination of azadirachtin residues in bovine muscle[J]. J Chromatogr Sci, 2011, 49(4): 327-331. doi: 10.1093/chrsci/49.4.327
[17] POZO O J, MARIN J M, SANCHO J V, et al. Determination of abamectin and azadirachtin residues in orange samples by liquid chromatography-electrospray tandem mass spectrometry[J]. J Chromatogr A, 2003, 992(1/2): 133-140. http://www.sciencedirect.com/science/article/pii/S002196730300325X
[18] 杨克武, 莫汉宏, 安凤春, 等.有机化合物水解的研究方法[J].环境化学, 1994, 13(3): 206-209. http://www.cnki.com.cn/Article/CJFDTOTAL-HJHX199403004.htm [19] 樊德方.农药残留分析与检测[M].上海:上海科学技术出版社, 1982: 8-26. [20] SHAUN J, DAVID E. Supercritical fluid extraction of oil and triterpenoids from neem seeds[J]. Phytochem Analysis, 1997, 8(5): 228-232. doi: 10.1002/(ISSN)1099-1565



 下载:
下载: