Cloning and sequence analysis of mRNA and promoter of pig FAM213B gene
-
摘要:目的
获得猪FAM213B基因完整mRNA和启动子序列,研究猪FAM213B基因表达,为探讨母猪妊娠的建立和胚胎发育调控机制奠定基础。落
方法通过5′RACE和3′RACE技术,获得基因完整mRNA序列,分析不同物种该基因氨基酸序列相似性;通过PCR克隆启动子区,并通过双荧光素酶报告基因载体系统转染猪子宫内膜细胞,研究其转录活性。
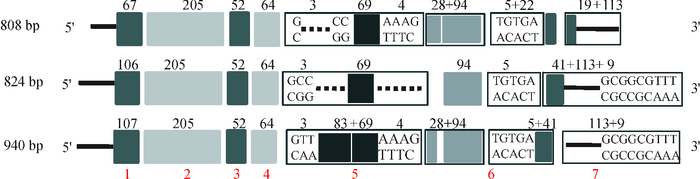
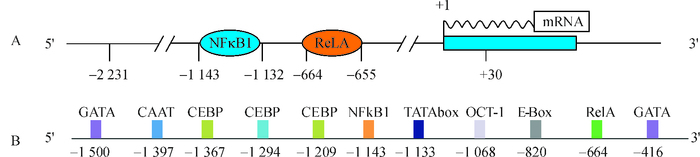
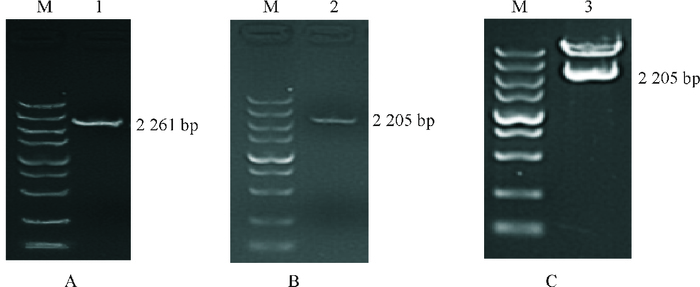
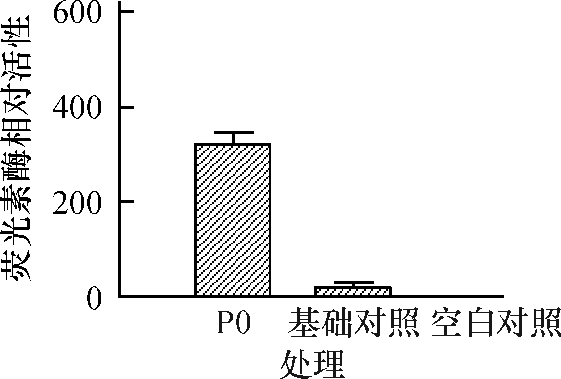
结果猪FAM213B基因mRNA全长808 bp,其中5′UTR、CDS区和3′UTR长度分别为67、609(含终止密码子)和132 bp(不含poly A序列),在17~106位氨基酸之间存在硫氧还蛋白折叠结构域;与猪FAM213B基因其他2个潜在转录本相比,三者都包含硫氧还蛋白折叠结构域,但蛋白三级结构存在较大差异;猪FAM213B氨基酸序列与山羊、牛和绵羊高度相似,相似性分别为94.03%、93.03%和91.54%。克隆获得2 261 bp(-2 231/+30) 的基因启动子序列,将其连接至双荧光素酶报告基因载体,转染猪子宫内膜细胞,发现获得的启动子片段能够启动下游报告基因的转录,在启动子区存在潜在的典型NFκB等转录因子结合位点。
结论本研究获得猪FAM213B基因转录本长度为808 bp,其蛋白存在硫氧还蛋白折叠功能结构域,其启动子序列(-2 231/+30) 在猪子宫内膜细胞中具有较强的转录活性。
Abstract:ObjectiveTo obtain the complete mRNA and promoter sequences of pig FAM213B gene, and provide a basis for studying the mechanism of the FAM213B gene in regulating gestation establishment and embryo development of female pigs.
MethodThe complete mRNA sequence of the FAM213B gene was obtained using 5′ and 3′RACE methods.The amino acid sequence similarities of different species were analyzed. The gene promoter was cloned, and its transcription activity was detected by the dual luciferase report system in porcine endometrial cells.
ResultThe mRNA of pig FAM213B gene was 808 bp in full length, including the 5′UTR, CDS and 3′UTR of 67, 609 (including the termination codon) and 132 bp (excluding poly A), respectively. A thioredoxin fold domain was predicted from the 17th to 106th amino acid residues. The obtained transcript and the other two computed transcripts all contained the thioredoxin fold domains, but the tertiary structures of three proteins were highly different. The amino acid sequence of pig FAM213B showed 94.03%, 93.03% and 91.54% similarities with those of goat, cattle and sheep, respectively. The cloned promoter sequence (-2 231/+30) of the FAM213B gene was linked with the dual luciferase report vector, and transfected into the endometrial cells. The promoter fragment could drive the expression of the downstream report gene. There were potential binding sites of transcription factors such as NFκB within the promoter.
ConclusionThe complete sequence of the transcript of pig FAM213B gene is 808 bp. The FAM213B protein contains a thioredoxin fold domain. The FAM213B promoter (-2 231/+30) has strong transcription activity in porcine endometrial cells.
-
Keywords:
- pig /
- FAM213B gene /
- transcription /
- promoter /
- cloning /
- endometrial cell /
- thioredoxin
-
-
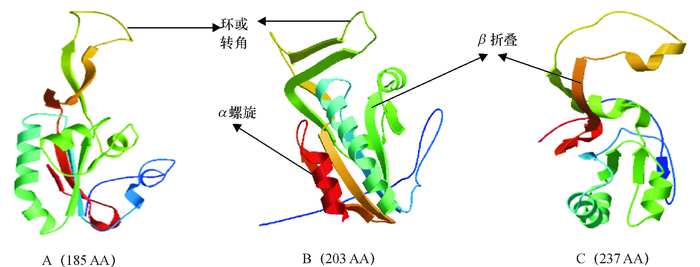
图 3 猪FAM213B基因不同转录本的蛋白质三级结构分析
A、B、C分别对应于图 2中3个转录本的蛋白结构,括号内数字表示3个转录本编码氨基酸数目。
Figure 3. The protein tertiary structures of three transcripts of porcine FAM213B gene
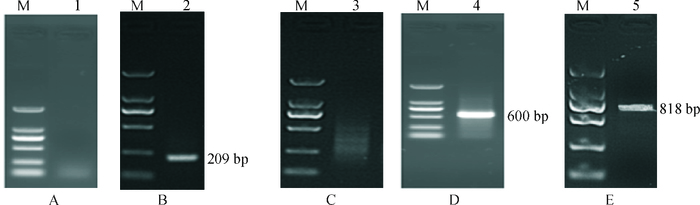
表 1 猪FAM213B基因mRNA全序列和启动子克隆所用引物信息
Table 1 The primers for cloning the mRNA and promoter of porcine FAM213B gene
名称 引物序列(5′→3′)1) 产物/bp 5′GSP1 GCGACACACCATGCAGCCGAAGC 5′GSP2 CACGGCCTCCCCGGTCACTGCGTGC 3′GSP1 TACGAAGCCTGTGGCAGGAGCAGG 3′GSP2 GACCAGCACGGCGTGCGCCTGGTGGG p-mRNA-F AGGACTGCAGGCGGAGAGGGCTG 609 p-mRNA-R CCAGGAACCACCCTTTAATGCT p-promoter-F ACCACCTGAGACTGTCGCCAAC 2 261(-2 231/+30) p-promoter-R GCAAGGTCCACCGTGCTCAT p0-promoter-F $\boxed{{\text{CG}}}\underline {{\text{ACGCGT}}} $ CGACCACCTGAGACTGTCG 2 005(-2 175/+30) p0-promoter-R $\boxed{{\text{CCC}}}\underline {{\text{AAGCTT}}}$ GGGCTCCGCACCAGCCCTCT 1) 黑框内为保护碱基,下划线为酶切位点;上游为Mlu I酶切位点,下游为Xho I酶切位点。 -
[1] MORIUCHI H, KODA N, OKUDA-ASHITAKA E, et al. Molecular characterization of a novel type of prostamide/prostaglandin F synthase, belonging to the thioredoxin-like superfamily[J]. J Biol Chem, 2008, 283(2): 792-801. doi: 10.1074/jbc.M705638200
[2] KOMOTO J, YAMADA T, WATANABE K, et al. Prostaglandin F2alpha formation from prostaglandin H2 by prostaglandin F synthase (PGFS): Crystal structure of PGFS containing bimatoprost[J]. Biochemistry, 2006, 45(7): 1987-1996. doi: 10.1021/bi051861t
[3] WACLAWIK A, ZIECIK A J. Differential expression of prostaglandin (PG) synthesis enzymes in conceptus during peri-implantation period and endometrial expression of carbonyl reductase/PG 9-ketoreductase in the pig[J]. J Endocrinol, 2007, 194(3): 499-510. doi: 10.1677/JOE-07-0155
[4] CHRISTENSON L K, FARLEY D B, Anderson L H, et al. Luteal maintenance during early pregnancy in the pig: Role for prostaglandin E2[J]. Prostaglandins, 1994, 47(1): 61-75. doi: 10.1016/0090-6980(94)90075-2
[5] ZIECIK A J. Old, new and the newest concepts of inhibition of luteolysis during early pregnancy in pig[J]. Domest Anim Endocrinol, 2002, 23(1/2): 265-275.
[6] GADSBY J E, LOVDAL J A, BRITT J H, et al. Prostaglandin F2 alpha receptor concentrations in corpora lutea of cycling, pregnant, and pseudopregnant pigs[J]. Biol Reprod, 1993, 49(3): 604-608. doi: 10.1095/biolreprod49.3.604
[7] WEEMS C W, WEEMSY S, RANDELR D. Prostaglandins and reproduction in female farm animals[J]. Vet J, 2006, 171(2): 206-228. doi: 10.1016/j.tvjl.2004.11.014
[8] BAZER F W, THATCHER W W, MATINAT-BOTTEF, et al. Composition of uterine flushings from Large White and prolific Chinese Meishan gilts[J]. Reprod Fertil Dev, 1991, 3(1): 51-60. doi: 10.1071/RD9910051
[9] ZHANG H, WANG S, LIU M, et al. Differential gene expression in the endometrium on gestation day 12 provides insight into sow prolificacy[J]. Bmc Genomics, 2013, 14: 45. doi: 10.1186/1471-2164-14-45
[10] SUZUKI-YAMAMOTO T, TOIDA K, SUGIMOTO Y, et al. Colocalization of prostaglandin F(2alpha) receptor FP and prostaglandin F synthase-I in the spinal cord[J]. J Lipid Res, 2009, 50(10): 1996-2003. doi: 10.1194/jlr.M800543-JLR200
[11] ZIECIK A J, WACLAWIK A, KACZMAREK M M, et al. Mechanisms for the establishment of pregnancy in the pig[J]. Reprod Domest Anim, 2011, 46(Suppl 3): 31-41.
[12] WACLAWIK A, JABBOUR H N, BLITEK A, et al. Estradiol-17beta, prostaglandin E2 (PGE2), and the PGE2 receptor are involved in PGE2 positive feedback loop in the porcine endometrium[J]. Endocrinology, 2009, 150(8): 3823-3832. doi: 10.1210/en.2008-1499
[13] KING A E, COLLINS F, KLONISCH T, et al. An additive interaction between the NFkappaB and estrogen receptor signalling pathways in human endometrial epithelial cells[J]. Hum Reprod, 2010, 25(2): 510-518. doi: 10.1093/humrep/dep421
[14] LINDSTROM T M, BENNETT P R. The role of nuclear factor kappaB in human labour[J]. Reproduction, 2005, 130(5): 569-581. doi: 10.1530/rep.1.00197
[15] LAIRD S M, TUCKERMAN E M, CORK B A, et al. Expression of nuclear factor kappaB in human endometrium; role in the control of interleukin 6 and leukaemia inhibitory factor production[J]. Mol Hum Reprod, 2000, 6(1): 34-40. doi: 10.1093/molehr/6.1.34
[16] MATHEW D J, SELLNER E M, GREEN J C, et al. Uterine progesterone receptor expression, conceptus development, and ovarian function in pigs treated with RU 486 during early pregnancy[J]. Biol Reprod, 2011, 84(1): 130-139. doi: 10.1095/biolreprod.110.086843
[17] INOUE J, GOHDA J, AKIYAMA T, et al. NF-kappaB activation in development and progression of cancer[J]. Cancer Sci, 2007, 98(3): 268-274. doi: 10.1111/cas.2007.98.issue-3



 下载:
下载: