Research progress on elongation of cooked rice
-
摘要:
水稻饭粒延伸性是指米粒蒸煮时的延伸特性,用蒸煮后米粒长度增加值与蒸煮前米粒长度的比值来衡量,是评价稻米蒸煮食味品质的重要指标之一。随着现代遗传学及基因组学相关理论和育种技术的发展,人们对水稻饭粒延伸性的遗传研究也日趋深入。本文综述了影响水稻饭粒延伸性的相关因素及其遗传研究进展,指出了水稻饭粒延伸性遗传研究目前存在的主要问题,分析了水稻饭粒延伸性遗传研究的应用前景。
Abstract:Cooked rice elongation (CRE) refers to the elongation characteristics of rice grains during cooking, is evaluated by the ratio of the added value of rice grain length after cooking to the length of rice grain before cooking. It is one of the important indicators of cooking and eating quality. With the development of modern genetics and genomics related theories and breeding technology, the genetic research of CRE has also become increasingly in-depth. In this paper, the related factors affecting CRE and the main progress of genetic research on CRE were summarized, the existing problems of genetic research on CRE were also pointed out, and the prospects of genetic research on CRE were analyzed.
-
敌草隆(Diuron),化学名称为3−(3,4−二氯苯基)−1,1−二甲基脲,是一种低毒、高效的取代脲类除草剂,广泛应用于棉花、甘蔗[1]、葡萄[2]等作物的苗前除草。该药还在新疆作为棉花脱叶剂(复配制剂噻苯·敌草隆)广泛应用于棉田机械化作业中[3]。然而,敌草隆对哺乳动物和鸟类有轻微毒性,对水生无脊椎动物中等毒性,在土壤和地下水中具有持久性[4]。在施药后70 d,径流水和土壤中仍可以检测到低浓度的敌草隆[5]。因此,研究敌草隆的降解对农业可持续发展具有重要意义。
随着社会经济发展,农药对环境的影响受到人们日益关注,Khongthon 等[6]在微米反应器中通过电化学高级氧化工艺降解敌草隆,降解率约为90%;Vanraes 等[7]优化了介质阻挡放电(Dieletric barrier discharge, DBD)等离子体反应器与活性炭纺织材料吸附相结合的装置,可以最大限度地减少敌草隆在水中的氧化副产物的生成。在农业可持续发展进程中,微生物降解农药作为一种生态友好型技术具有很好的应用前景[8],生物修复环境中残留的敌草隆是目前最佳途径之一。据报道,目前分离得到的降解敌草隆的菌株主要为白腐真菌(Phanerochaete chrysosporium[9]、Ganoderma lucidum[10]、Trametes versicolor[11]、),费氏埃希菌Escherichia fergusonii[12]及嗜根寡养单胞菌Stenotrophomonas rhizophila[13]。本文从长期施用敌草隆的棉田土壤中筛选分离出降解效果好的敌草隆降解菌株,并进行形态学观察、生理生化及分子生物学鉴定,探索菌株的降解特性及优化降解条件,提高菌株降解效率,以期为该菌剂的制备提供理论基础,对敌草隆污染土壤修复提供理论依据。
1. 材料与方法
1.1 供试材料
1.1.1 仪器
超净工作台;高速冷冻离心机;生化培养箱;高压灭菌锅;PCR仪;紫外分光光度计;高效液相色谱仪(Waters e2695 Separations Module);旋转蒸发仪;恒温水浴锅;摇床;超纯水器;精确电子分析天平;烘箱;震荡仪。
1.1.2 土壤采集及制备
2018年9月在新疆生产建设兵团第八师147团和149团采集连续施用敌草隆药剂10年以上的棉田,取土深度10~20 cm,各采集1 kg。将采集的土样去除大粒石块并过2 mm筛,风干后贴标存于密封袋,置于4 ℃冰箱保存。
1.1.3 药剂及培养基
药剂:敌草隆原药、敌草隆标准品(质量分数98.8%)购于Dr.Ehrenstorfer公司;细菌基因组DNA提取试剂盒购于北京全式金生物技术有限公司;甲醇(分析纯)、二氯甲烷(分析纯)购于天津市富余精细化工有限公司;无水硫酸钠(分析纯)购于天津市盛奥化学试剂有限公司。
液体培养基:无机盐培养基( MgSO4·7H2O 0.2 g,KH2PO4 0.5 g,(NH4)2SO4 1 g,NaCl 0.5 g,K2HPO4 1.5 g,蒸馏水1000 mL,pH 7.0~7.2),牛肉膏蛋白胨培养基(牛肉膏3.0 g,蛋白胨 10.0 g,NaCl 5.0 g,蒸馏水1000 mL,pH 7.0~7.2),富集培养基(在无机盐培养基中添加所需含量的敌草隆),LB培养基(胰蛋白胨10 g,酵母提取物5 g,NaCl 10 g,蒸馏水1000 mL);固体培养基均在以上液体培养基中加入18 g/L琼脂粉末,高氏一号培养基(可溶性淀粉20 g,KNO3 1 g,K2HPO4 0.5 g,MgSO4 0.5 g,FeSO4 0.01 g)。
1.2 试验方法
1.2.1 敌草隆降解菌株的筛选
在含100 mg/L敌草隆的90 mL富集培养液中加入10 g供试土样,在30 ℃、180 r/min下培养7 d后,取体积分数为10%的混合液体,接种到含500 mg/L敌草隆的富集培养液中继续培养7 d后,再按照10%(φ)的接菌量将上述富集培养液接种到含1000 mg/L敌草隆的富集培养液中,如此持续不断转接至1500、1800、2000 mg/L时,进行平板涂布,观察菌落形成情况(2000 mg/L时未形成菌落)。
随机从1800 mg/L富集培养液的平板中挑取36组形态不同且清晰的菌落通过划线处理的方法接种于含100 mg/L敌草隆的牛肉膏蛋白胨固体培养基上,于30 ℃恒温培养箱培养。待平板长出单个菌落,直至培养基中敌草隆质量浓度为2000 mg/L(进行压力筛选),取7株生长良好的菌株编号并放入4 ℃的冰箱内进行保存。
从以上7株菌落中挑取单菌落接入LB液体培养基中,30 ℃、180 r/min下制成菌液。在含100 mg/L敌草隆的无机盐降解培养基中加入10%体积的菌液,置于摇床上,分别摇培3、6、9、12、15 d后,HPLC法检测降解液中敌草隆的含量,计算降解率(重复3次,设置无菌对照)。
1.2.2 敌草隆的HPLC检测
取降解液2 mL于管内,12000 r/min离心处理8 min,取上层液体1 mL与二氯甲烷4 mL,充分震荡后分层,取下层有机相至无水硫酸钠柱中,将过柱后的液体移至圆底烧瓶,减压浓缩至接近干燥,加入甲醇定容至2 mL,再通过有机相微孔过滤器过滤后装入样瓶,待液相色谱检测。
色谱条件:色谱柱5.0 μm×4.6 mm×250 mm、流动相V(甲醇)∶V(水)=60∶40、流速1.0 mL/min、柱温 35 ℃、测定波长254 nm、进样量20 μL。
1.2.3 敌草隆降解菌株的鉴定
将降解率最高的菌株接种至LB平板上,30 ℃条件下培养,进行形态观察、生理生化试验(生理生化鉴定参照BIOLOG GEN III、API 20 E、API 20 NE等说明书,其中BIOLOG GEN III鉴定板用于革兰阴性和阳性好氧菌的鉴定,API 20E是肠杆菌科和其他G−杆菌的标准鉴定系统,API 20NE是鉴定不属肠杆菌科、革兰阴性非苛养杆菌的标准鉴定系统)。
选择新鲜菌体作为PCR扩增模板进行分子鉴定,采用细菌16S rRNA序列通用引物27F(5′-AGAGTTTGATCCTGGCTCAG-3′)和1492-R(5′-GGTTACCTTGTTACGACTT-3′)扩增菌株目标片段。PCR反应体系(100 μL):正、反向引物(10 μmol/L)各2 μL;Taq(5 U/μL)0.8 μL;10×PCR Buffer(Mg2+Plus)10 μL;dNTP Mixture(各2.5 mmol/L)8 μL;模板DNA 2.5 ng;ddH2O补足到100 μL。PCR反应条件:95 ℃ 5 min;95 ℃ 1 min,57 ℃ 1 min,72 ℃ 1.5 min,30个循环;72 ℃ 5 min。
使用引物nrdA-F (5′-ACTGATTCCCGACCTGTTC-3′)和nrdA-R(5′-TTCGATTTGACGTACAAGTTCTGG-3′)扩增菌株目标片段。nrdA PCR反应体系(100 μL):正、反向引物(50 μmol/L)各2 μL;Taq(2.5 U/μL)0.8 μL;10×PCR Buffer (Mg2+Plus)10 μL;dNTP Mixture(各2.5 mmol/L)8 μL;模板DNA 10 ng;ddH2O补足到100 μL。nrdA PCR反应条件: 95 ℃ 5 min;95 ℃ 45 s,52 ℃ 45 s,72 ℃ 60 s,33个循环;72 ℃ 10 min。
产物进行测序后,将结果在GenBank数据库中通过BLAST进行比对,采用临位连接法构建系统进化树。
1.2.4 不同培养条件对菌株降解率的影响
探索降解菌在不同敌草隆初始质量浓度、接菌量、蔗糖含量、pH、温度及最优条件下对敌草隆的降解效果,评估降解能力,其中敌草隆初始质量浓度为25、50、100、200和500 mg/ L;接菌量为1%、3%、5%、10%和15%(φ);蔗糖质量分数为0.0%、0.5%、1.0%、1.5%和2.0%;pH为5.0、6.0、7.0、8.0和9.0;温度为20、25、30、35和40 ℃。
1.3 数据统计与分析
试验结果均采用SPSS 22.0与Excel统计软件进行数据方差分析,运用Duncan’s法进行各处理间的多重比较分析,本试验所显示的结果均为3次重复测定的平均值。
2. 结果与分析
2.1 HPLC检测敌草隆的标准曲线
用敌草隆标准品分别配置20、50、100、200、500、1000 mg/L的敌草隆样品,进行HPLC检测,以峰面积为纵坐标,添加水平为横坐标绘制标准曲线,并进行线性回归分析,获得的标准曲线为:y=4.13×104x+1.81×105(R2=0.99)(图1),相对保留时间为5.658 min(图2)。
2.2 HPLC检测方法的准确性和精密度
敌草隆在空白培养基中的添加质量浓度分别为25、50、100 mg/L,进行HPLC检测,每个质量浓度重复5次,计算回收率和相对标准偏差(RSD)。测定的样品中敌草隆的回收率为94.72%~96.99%,RSD为1.29%~1.86%(表1),符合对敌草隆的残留检测要求,可用于培养基中敌草隆残留检测。
表 1 敌草隆在培养基中的回收率Table 1. The recovery rate of diuron in mediumρ添加/(mg·L−1)
Added concentration回收率/% Recovery rate 相对标准偏差/%
Relative standard deviation (RSD)1 2 3 4 5 平均 Average 25 92.82 96.37 91.23 98.58 94.62 94.72 1.29 50 96.92 90.78 100.02 91.69 92.29 94.34 1.77 100 91.47 102.76 98.22 95.18 97.32 96.99 1.86 2.3 敌草隆降解菌的降解速率测定
经平板初筛,逐步提高培养基中敌草隆的含量进行压力筛选,筛选出7株菌株并对其命名为SL-6、SL-7、SL-8、SL-9、SL-10、SL-11和SL-12,涂板保存。初筛获得的7株菌株,在含100 mg/L敌草隆、接菌量10%(φ)的培养基中,30℃、180 r/min的条件下进行培养。
菌株SL-6、7、9对敌草隆的降解效果明显高于其余4株。且在第15天,降解率均高达90%以上(图3),因此选取该3株菌株开展后续试验。
在敌草隆质量浓度为100 mg/L的培养基中,以10%的接菌量(φ)分别接种初筛获得3个菌株后,敌草隆的消解动态符合消解动力学方程。如表2所示,菌株SL-6、7、9对培养基中的敌草隆消解方程分别为ρt = 74.482e−0.213t、ρt = 59.264e−0.166t、ρt = 58.165e−0.182t,其中ρt为t时刻敌草隆的质量浓度;半衰期分别为3.2、4.2、3.8 d。
表 2 敌草隆的消解动力学方程和相关参数Table 2. Digestion kinetic equation and related parameters of diuron菌株
Strain消解方程
Degradation equation速率常数
Rate constant (K)决定系数
Coefficient of determination (R2)t半衰/d
Half-lifeSL-6 ρt = 74.482e−0.213t 0.213 0.9636 3.2 SL-7 ρt = 59.264e−0.166t 0.166 0.9782 4.2 SL-9 ρt = 58.165e−0.182t 0.182 0.9691 3.8 2.4 降解菌株的鉴定
分别提取3株敌草隆降解菌株的DNA,并将其作为模板,利用16S rRNA基因的通用引物进行PCR扩增,分别得到1500 bp左右的片段,将PCR产物进行回收连接转化,然后进行测序。将菌株SL-6、7、9的16S rDNA基因序列在GeneBank中进行BLAST比对,结果发现,菌株SL-6、7、9与变形菌门Proteobacteria的Achromobacter xylosoxidans菌株的16S rDNA序列相似度均高达99.94%以上,由此首先确定菌株SL-6、7、9均为无色杆菌属Achromobacter,因此选取该3株菌种降解率最高(94.6%)的SL-6进行后续试验及进一步鉴定,并将菌株SL-6鉴定至种。
2.5 菌株SL-6的鉴定
2.5.1 菌株SL-6形态学鉴定
菌株SL-6的LB培养基菌落形态为白色,其单菌落形态呈白色且表面隆起,外表光滑湿润,边缘光滑平整不透明,有黏性;菌株SL-6的革兰氏染色呈阴性(图4)。
2.5.2 菌株SL-6的生理生化特征
菌株SL−6细胞形态呈杆状;经BIOLOG GEN III鉴定反应呈阳性的包括氧化酶、接触酶、对羟基苯乙酸、糊精、丙酮酸甲酯、柠檬酸、1%乳酸钠、林可霉素、萘啶酸、夫西地酸、十四烷硫酸钠、亚碲酸钾、葡糖醛酰胺、丙酸、乙酸、糖质酸、醋竹桃霉素、万古霉素、氨曲南、利福霉素、二甲胺四环素、四唑紫、四唑蓝、丁酸钠、α−D−葡萄糖、D−果糖、L−丙氨酸、L−乳酸、L−天冬氨酸、L−谷氨酸、α−酮戊二酸、D−苹果酸、L−鼠李糖、L−焦谷氨酸、L−苹果酸、L−丝氨酸、D−半乳糖醛酸、γ−氨基丁酸、α−羟丁酸、D−葡糖酸、β−羟基−D,L−丁酸、D−葡萄糖醛酸、α−丁酮酸、D−果糖−6−磷酸,反应呈弱阳性的包括溴代丁二酸、盐酸胍、氯化锂、黏酸、甲酸、溴酸钠、D−岩藻糖、L−组氨酸、D−天冬氨酸,反应呈阴性的包括明胶、龙胆二糖、蔗糖、松二糖、水苏糖、肌苷、果胶、吐温−40、肌醇、甘油、乙酰乙酸、奎宁酸、D−甘露糖、甘氨酸−L−脯氨酸、D−麦芽糖、D−乳酸甲酯、D−海藻糖、D−半乳糖、L−精氨酸、D−纤维二糖、3−甲基−D−葡萄糖、L−岩藻糖、D−丝氨酸、D−棉子糖、D−山梨醇、α−D−乳糖、D−甘露醇、D−蜜二糖、D−阿糖醇、L−半乳糖酸内酯、D−水杨苷、N−乙酰−D−葡糖胺、N−乙酰−β−D−甘露糖胺、N−乙酰−D−半乳糖胺、N−乙酰神经氨酸;API 20E鉴定反应呈阳性的包括精氨酸双水解酶、柠檬酸、丙酮酸盐,反应呈阴性的包括赖氨酸脱羧酶、鸟氨酸脱羧酶、硫代硫酸钠、脲酶、色氨酸脱氨酶、色氨酸、明胶;API 20NE鉴定反应呈阳性的包括硝酸钾,反应呈阴性的包括色氨酸、葡萄糖、精氨酸、脲素、七叶灵、明胶、β−半乳糖苷酶。以上生理生化特征所包含的资料库无法将其鉴定至种,故后续通过16S rDNA与nrdA基因序列进行分析鉴定。
2.5.3 菌株SL-6与相关菌种的16S rDNA序列系统发育进化树
采用临位连接法显示菌株SL-6与相关菌种的16S rDNA系统发育树,进行1000次的相似度重复计算,菌株SL-6与木糖氧化无色杆菌A. xylosoxidans的相似性最高,为100%。(图5)。
2.5.4 菌株SL-6与相关菌种的nrdA基因序列系统发育进化树
采用临位连接法显示菌株SL-6与相关菌种的nrdA基因序列系统发育树,进行1000次的相似度重复计算,图中发育树节点只显示Bootstrap值大于50%数值。菌株SL-6与木糖氧化无色杆菌A. xylosoxidans的nrdA基因序列相似性高达99.996%(图6)。
2.6 SL-6菌株不同培养条件对敌草隆降解率的影响
2.6.1 敌草隆质量浓度对敌草隆降解率的影响
降解率随着敌草隆初始质量浓度的增加呈逐渐增加后减少的趋势(图7A)。当培养液中敌草隆质量浓度为200 mg/L时,降解效果最佳,第3天的降解率高达82.2%;当质量浓度为25 mg/L时,第3天的降解率为39.4%,仅为200 mg/L质量浓度的47.9%。
![]() 图 7 不同培养条件对菌株SL-6降解敌草隆的影响A:敌草隆初始质量浓度;B:接菌量;C:蔗糖含量;D:pH;E:温度;F:最优条件;图中数据为平均值和标准误(n=3);各图中,柱子上方的不同小写字母表示差异显著(P < 0.05, Duncan’s法)Figure 7. Effect of various cultural conditions on diuron degradation by strain SL-6A: Initial concentration of diuron; B: Inoculation amount; C:Sucrose content; D: pH; E: Temperature; F: Optimal conditions;Data in the figure are means and standard errors (n=3); In each graph, different lowercase letters on bars indicate significant differences(P < 0.05, Duncan’s method)
图 7 不同培养条件对菌株SL-6降解敌草隆的影响A:敌草隆初始质量浓度;B:接菌量;C:蔗糖含量;D:pH;E:温度;F:最优条件;图中数据为平均值和标准误(n=3);各图中,柱子上方的不同小写字母表示差异显著(P < 0.05, Duncan’s法)Figure 7. Effect of various cultural conditions on diuron degradation by strain SL-6A: Initial concentration of diuron; B: Inoculation amount; C:Sucrose content; D: pH; E: Temperature; F: Optimal conditions;Data in the figure are means and standard errors (n=3); In each graph, different lowercase letters on bars indicate significant differences(P < 0.05, Duncan’s method)2.6.2 菌株不同接菌量对敌草隆降解率的影响
随着接菌量增大,敌草隆的降解率也随之增高,当接菌量(φ)为15%时,降解率为63.5%,但接菌量在5%~15%之间,降解率的提高并不显著(图7B)。
2.6.3 外加碳源含量对敌草隆降解率的影响
向培养液中分别加入质量分数为0.5%、1.0%、1.5%、2.0%的蔗糖作为外加碳源(图7C),3 d后敌草隆的降解率并未有较大变化。与不添加碳源相比,添加0.5%~2.0%的蔗糖碳源,降解率在40.6%~53.2%之间,均低于不添加碳源的57.4%降解率。
2.6.4 培养液pH对敌草隆降解率的影响
分析培养液pH对菌株SL-6降解率影响,结果表明,pH 8时降解率最高,为59.6%(图7D)。
2.6.5 培养温度对敌草隆降解率的影响
分析不同培养温度对菌株SL-6降解率的影响,结果表明,20~40 ℃时的降解率呈现逐渐升高后降低的趋势,温度为30℃时,降解效果最佳,第3天的降解率高达57.2%;当温度为20 ℃时,第3天的降解率为18.2%,仅为30℃的31.8%(图7E)。
2.6.6 最优培养条件下菌株降解率
最优敌草隆初始质量浓度为200 mg/L、接菌量为15%(φ)、pH为8、培养温度为30℃,该条件下,分别于1、2、3、4、5 d后,运用液相色谱法检测培养液中敌草隆的剩余浓度,并计算降解率。菌株SL-6在最优培养条件下,随着时间的增加,敌草隆的降解率亦随之增高,第5天的降解率为93.1%;第1天的降解率为43.1%,仅为第5天的46.3%(图7F)。
3. 讨论与结论
在连续施用敌草隆10年以上的棉田中分离得到降解菌,通过形态学观察、生理生化测试、16S rDNA及nrdA基因序列相似性分析,鉴定菌株SL-6为木糖氧化无色杆菌A. xylosoxidans。本研究结果表明:菌株SL-6在敌草隆初始质量浓度为200 mg/L时,降解率为82.2%;接菌量为15%(φ)时,降解率为63.5%;pH为8时,降解率为59.6%;温度为30 ℃时,降解率为57.2%;与不添加蔗糖相比,添加蔗糖后降解率均低于57.4%。于上述最优培养条件下,在第5天,菌株SL-6对200 mg/L的敌草隆降解率达93.1%。
本研究分离得到1株高效降解敌草隆的菌株,为木糖氧化无色杆菌,是一种毒力较弱的革兰阴性杆菌。该菌能够引起轻微的菌血症、心包炎、导管相关的血流感染、肺炎等[14],相较而言,该菌株治理环境污染时表现出极大潜力,在合理规范的操作下,引起人体不适微乎其微,有研究表明,该菌株可通过碳酸盐矿化作用固定土壤中的复合重金属(Cu、Pb、Cd)[15]以及固结土壤中的有效态Cd,从而减少水稻对Cd的吸收[16]。
国内外已有可降解敌草隆的部分真菌和细菌,以及对降解条件进行优化的报道。孙纪全等[17]报道了1株细菌菌株Y57,鉴定为鞘氨醇单胞菌属Sphingomonas sp.,该菌株在敌草隆初始质量浓度为20 mg/L,30℃及5%(φ)接菌量时,其降解率达到80%以上,与该研究相比,本研究所得降解条件中敌草隆初始质量浓度更高,接菌量更大,培养温度相同,但降解率相对较高。封国君等[18]报道了1株真菌菌株D12,鉴定为镰刀菌属Fusarium,该菌株在敌草隆初始质量浓度为50 mg/L,35 ℃及pH 6.0时,经过7 d培养,降解率为57.42%,半衰期为7.30 d,与该研究相比,本研究所得降解条件为碱性环境,培养温度更低,敌草隆初始质量浓度更高,同时降解率相对更高。杨孟然[19]报道了敌草隆降解菌株D47(Arthrobacter globiformis),处理4 d后该菌株对0.5~20.0 mg/L的敌草隆降解率均为100.0%,与该研究相比,本研究所得降解条件中敌草隆初始质量浓度更高,但处理时间与降解率相对较低。以上学者报道的降解菌株在降解过程中敌草隆初始质量浓度较低,而本研究中菌株SL-6可在敌草隆初始质量浓度较高的环境中对其进行降解,无需外加碳源,适于敌草隆残留极端的环境条件,同时菌株SL-6降解敌草隆的最适温度在30 ℃,该温度下无需供给额外能量,此外,菌株SL-6只有一定的耐弱碱性,而新疆棉田土壤呈一定的弱碱性,为该菌株的生产应用提供条件。菌株SL-6的降解能力与pH及温度等因素密切相关,还发现在接菌量较低的情况下,其对敌草隆的降解能力大大减弱。经过系列分析推测,该菌株降解敌草隆时,产生的酶的活性不是特别高,后期应进一步优化。
迄今为止,对于敌草隆降解菌的研究大部分集中于降解特性,关于降解机制的相关研究较少。敌草隆的主要代谢产物为3,4−二氯苯胺(DCA)、3−(3,4−二氯苯基)脲(DCPU )、3−(3,4−二氯苯基)−1−甲基脲(DCPMU )[20]。变色栓菌[21] Trametes versicolor培养7 d后,除降解敌草隆(降解率为83%)外,主要代谢产物DCA(降解率为100%)迅速降解,在降解过程中,根据鉴定的转化产物,3−(3,4−二氯苯基)−1−羟甲基−1−甲基脲(DCPHMU)代谢为N′端去烷基化合物DCPMU和DCPU,DCPHMU的发现揭示了羟基化对N′端去甲基化的相关作用,有助于更好地研究敌草隆降解机制的反应机理。
-
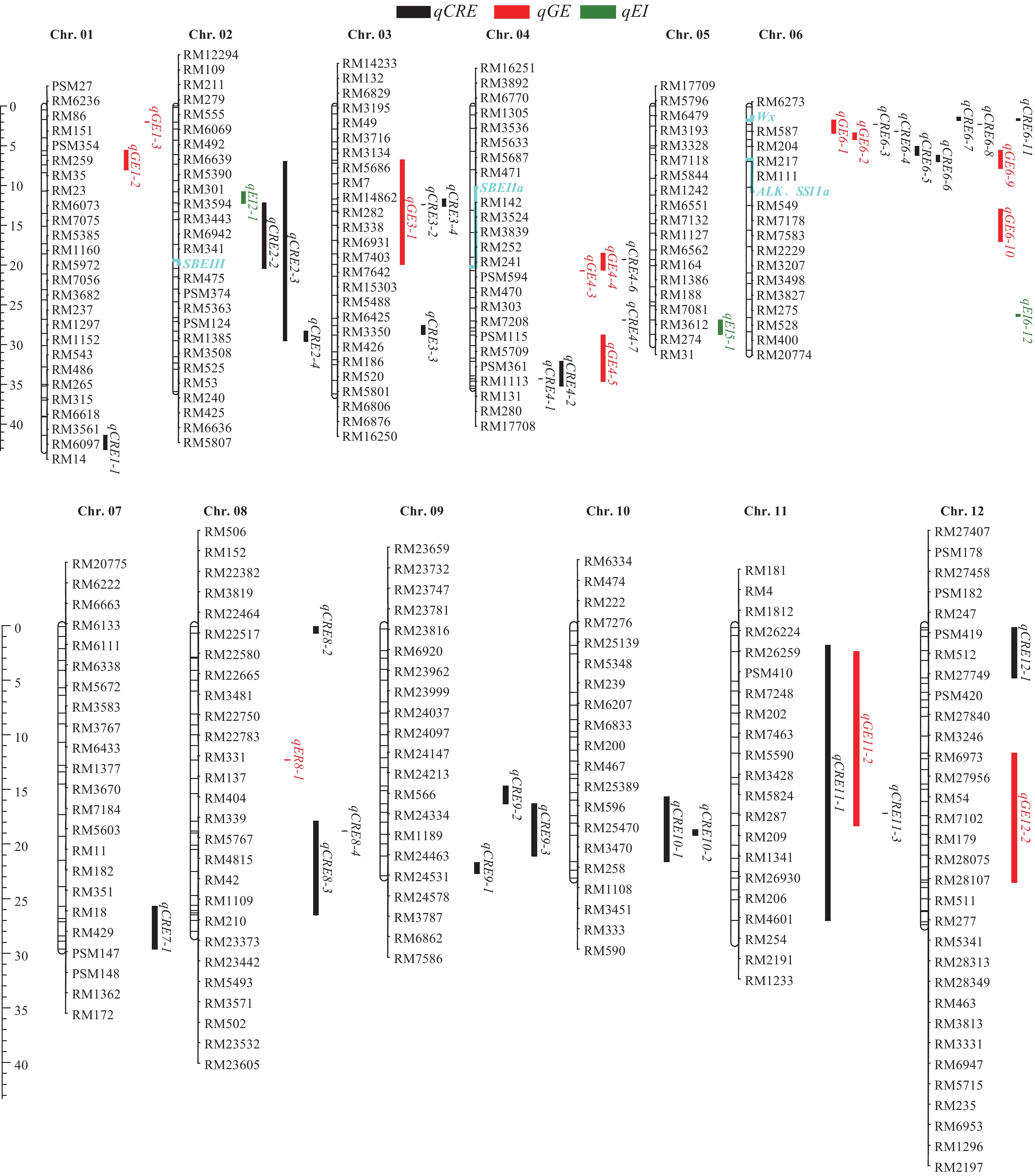
图 2 部分已鉴定的水稻饭粒延伸性QTLs
Chr.:染色体;黑色、红色和绿色条块分别代表饭粒延伸性、饭粒伸长率和米粒延伸指数的QTL,条块位置代表QTL的估计位置;蓝色为已克隆淀粉相关基因的物理位置
Figure 2. Partial identified QTLs for cooked rice elongation
Chr.: Chromosome; The bars in black, red, and green represent QTLs for cooked rice elongation, cooked rice elongation rate, and rice grain elongation index, respectively, the location of the bar represents the estimated location of the QTL; Blue is the physical location of the cloned starch-related genes
-
[1] SINGH N, KAUR L, SODHI N S, et al. Physicochemical, cooking and textural properties of milled rice from different Indian rice cultivars[J]. Food Chemistry, 2005, 89(2): 253-259. doi: 10.1016/j.foodchem.2004.02.032
[2] 张桂权. 5G水稻的演变和发展[J]. 华南农业大学学报, 2019, 40(5): 211-216. doi: 10.7671/j.issn.1001-411X.201905075 [3] 袁隆平. 超级杂交水稻育种研究新进展[J]. 中国农村科技, 2010(Z1): 24-25. doi: 10.3969/j.issn.1005-9768.2010.02.006 [4] 方志强, 陆展华, 王石光, 等. 稻米品质性状研究进展与应用[J]. 广东农业科学, 2020, 47(5): 11-20. doi: 10.16768/j.issn.1004-874X.2020.05.002 [5] 莫惠栋. 我国稻米品质的改良[J]. 中国农业科学, 1993, 26(4): 8-14. [6] 王慧, 张从合, 陈金节, 等. 稻米品质性状影响因素及相关基因研究进展[J]. 中国稻米, 2018, 24(4): 16-21. doi: 10.3969/j.issn.1006-8082.2018.04.004 [7] 程鸿燕, 韩渊怀. 大米食味品质的研究及其育种进展[J]. 山西农业大学学报(自然科学版), 2016, 36(12): 890-896. doi: 10.13842/j.cnki.issn1671-8151.2016.12.023 [8] DOU Z, TANG S, CHEN W, et al. Effects of open-field warming during grain-filling stage on grain quality of two japonica rice cultivars in lower reaches of Yangtze River delta[J]. Journal of Cereal Science, 2018, 81: 118-126. doi: 10.1016/j.jcs.2018.04.004
[9] 何予卿, 邢永忠, 葛小佳, 等. 水稻米饭延伸指数相关性状的基因定位研究[J]. 分子植物育种, 2003, 1(5/6): 613-619. doi: 10.3969/j.issn.1672-416X.2003.05.004 [10] KHUSH G S, PAULE C M, CRUZ N D. Rice grain quality evaluation and improvement at IRRI[M]//Chemical Aspects of Rice Grain Quality. Los Baños, Laguna, Philippines: Proceedings of a Workshop, International Rice Research Institute, 1979: 21-31.
[11] 汤圣祥. 我国杂交水稻蒸煮与食用品质的研究[J]. 中国农业科学, 1987, 20(5): 17-22. [12] TAN Y F, XING Y Z, LI J X, et al. Genetic bases of appearance quality of rice grains in Shanyou 63, an elite rice hybrid[J]. Theoretical and Applied Genetics, 2000, 101(5): 823-829.
[13] TIAN Z, QIAN Q, LIU Q, et al. Allelic diversities in rice starch biosynthesis lead to a diverse array of rice eating and cooking qualities[J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(51): 21760-21765. doi: 10.1073/pnas.0912396106
[14] JULIANO B O, PEREZ C M. Results of a collaborative test on the measurement of grain elongation of milled rice during cooking[J]. Journal of Cereal Science, 1984, 2(4): 281-292. doi: 10.1016/S0733-5210(84)80016-8
[15] VIVEKANADAN P, GIRIDHARAN S. Genetic variability and character association for kernel and cooking quality traits in rice[J]. Oryza, 1998, 35(3): 242-245.
[16] 包劲松, 谢建坤, 夏英武. 籼稻米粒延伸性的遗传研究[J]. 作物学报, 2001, 27(4): 489-492. doi: 10.3321/j.issn:0496-3490.2001.04.014 [17] LI J M, XIAO J H, GRANDILLO S, et al. QTL detection for rice grain quality traits using an interspecific backcross population derived from cultivated Asian (O. sativa L. ) and African (O. glaberrima S. ) rice[J]. Genome, 2004, 47(4): 697-704. doi: 10.1139/g04-029
[18] 张光恒, 曾大力, 郭龙彪, 等. 水稻米粒延伸性的遗传剖析[J]. 遗传, 2004, 26(6): 887-892. doi: 10.3321/j.issn:0253-9772.2004.06.021 [19] 沈圣泉, 庄杰云, 王淑珍, 等. 水稻米粒延伸性QTLs定位和基因型与环境互作分析[J]. 中国水稻科学, 2005, 19(4): 319-322. doi: 10.3321/j.issn:1001-7216.2005.04.006 [20] GE X J, XING Y Z, XU C G, et al. QTL analysis of cooked rice grain elongation, volume expansion, and water absorption using a recombinant inbred population[J]. Plant Breeding, 2005, 124(2): 121-126. doi: 10.1111/j.1439-0523.2004.01055.x
[21] TIAN R, JIANG G, SHEN L, et al. Mapping quantitative trait loci underlying the cooking and eating quality of rice using a DH population[J]. Molecular Breeding, 2005, 15(2): 117-124. doi: 10.1007/s11032-004-3270-z
[22] 陆贤军, 康海岐, 姜华, 等. 水稻核心种质及成恢448回交后代的稻米延伸性研究[J]. 分子植物育种, 2005, 3(5): 676-680. doi: 10.3969/j.issn.1672-416X.2005.05.014 [23] WANG Y, LI J. Genes controlling plant architecture[J]. Current Opinion in Biotechnology, 2006, 17(2): 123-129. doi: 10.1016/j.copbio.2006.02.004
[24] 康海岐, 陆贤军, 高方远, 等. 成恢448与Basmati 370回交后代的米粒延伸性遗传和相关分析[J]. 作物学报, 2006, 32(9): 1361-1370. doi: 10.3321/j.issn:0496-3490.2006.09.016 [25] AMARAWATHI Y, SINGH R, SINGH A K, et al. Mapping of quantitative trait loci for basmati quality traits in rice (Oryza sativa L. )[J]. Molecular Breeding, 2007, 21(1): 49-65. doi: 10.1007/s11032-007-9108-8
[26] WANG L Q, LIU W J, XU Y, et al. Genetic basis of 17 traits and viscosity parameters characterizing the eating and cooking quality of rice grain[J]. Theoretical and Applied Genetics, 2007, 115(4): 463-476. doi: 10.1007/s00122-007-0580-7
[27] 姜树坤, 黄成, 徐正进, 等. 粳稻米粒延伸性的QTL剖析[J]. 植物生理学报, 2008, 44(6): 1091-1094. doi: 10.13592/j.cnki.ppj.2008.06.022 [28] LIU L L, YAN X Y, JIANG L, et al. Identification of stably expressed quantitative trait loci for cooked rice elongation in non-Basmati varieties[J]. Genome, 2008, 51(2): 104-112. doi: 10.1139/G07-106
[29] GOVINDARAJ P, VINOD K K, ARUMUGACHAMY S, et al. Analysing genetic control of cooked grain traits and gelatinization temperature in a double haploid population of rice by quantitative trait loci mapping[J]. Euphytica, 2009, 166(2): 165-176. doi: 10.1007/s10681-008-9808-0
[30] 沈年伟, 来凯凯, 粘金沯, 等. 稻米出饭特性QTL分析及遗传研究[J]. 中国水稻科学, 2011, 25(5): 475-482. doi: 10.3969/j.issn.1001-7216.2011.05.004 [31] SWAMY B P M, KALADHAR K, RANI N S, et al. QTL analysis for grain quality traits in 2 BC2F2 populations derived from crosses between Oryza sativa cv Swarna and 2 accessions of O. nivara[J]. Journal of Heredity, 2012, 103(3): 442-452. doi: 10.1093/jhered/esr145
[32] HOSSEINI M, HOUSHMAND S, MOHAMADI S, et al. Detection of QTLs with main, epistatic and QTL × environment interaction effects for rice grain appearance quality traits using two populations of backcross inbred lines (BILs)[J]. Field Crops Research, 2012, 135: 97-106. doi: 10.1016/j.fcr.2012.07.009
[33] YANG D, ZHANG Y, ZHU Z, et al. Substitutional mapping the cooked rice elongation by using chromosome segment substitution lines in rice[J]. Molecular Plant Breeding, 2013, 4: 107-115.
[34] CHENG A, ISMAIL I, OSMAN M, et al. Mapping of quantitative trait loci for aroma, amylose content and cooked grain elongation traits in rice[J]. Plant Omics Journal, 2014, 7(3): 152-157.
[35] RATHI S, PATHAK K, YADAV R N S, et al. Association studies of dormancy and cooking quality traits in direct-seeded indica rice[J]. Journal of Genetics, 2014, 93(1): 3-12. doi: 10.1007/s12041-014-0319-6
[36] LI Y, TAO H, XU J, et al. QTL analysis for cooking traits of super rice with a high-density SNP genetic map and fine mapping of a novel boiled grain length locus[J]. Plant Breeding, 2015, 134(5): 535-541. doi: 10.1111/pbr.12294
[37] OKPALA N E, DUAN L, SHEN G, et al. Identification of putative metabolic biomarker underlying cooked rice elongation[J]. Plant Omics, 2017, 10(3): 164-168. doi: 10.21475/poj.10.03.17.pne670
[38] SINGH V, SINGH A K, MOHAPATRA T, et al. Pusa Basmati 1121: A rice variety with exceptional kernel elongation and volume expansion after cooking[J]. Rice, 2018, 11: 19. doi: 10.1038/s41598-019-44856-2
[39] ARIKIT S, WANCHANA S, KHANTHONG S, et al. QTL-seq identifies cooked grain elongation QTLs near soluble starch synthase and starch branching enzymes in rice (Oryza sativa L.)[J]. Scientific Reports, 2019, 9: 8328.
[40] KATO K, SUZUKI Y, HOSAKA Y, et al. Effect of high temperature on starch biosynthetic enzymes and starch structure in japonica rice cultivar ‘Akitakomachi’ (Oryza sativa L.) endosperm and palatability of cooked rice[J]. Journal of Cereal Science, 2019, 87: 209-214. doi: 10.1016/j.jcs.2019.04.001
[41] OKPALA N E, POTCHO M P, AN T Y, et al. Low temperature increased the biosynthesis of 2-AP, cooked rice elongation percentage and amylose content percentage in rice[J]. Journal of Cereal Science, 2020, 93: 102980. doi: 10.1016/j.jcs.2020.102980
[42] 岳红亮, 赵庆勇, 赵春芳, 等. 江苏省半糯粳稻食味品质特征及其与感官评价的关系[J]. 中国粮油学报, 2020, 35(6): 7-14. doi: 10.3969/j.issn.1003-0174.2020.06.002 [43] QIU X, YANG J, ZHANG F, et al. Genetic dissection of rice appearance quality and cooked rice elongation by genome-wide association study[J]. The Crop Journal, 2021, 9(6): 1470-1480. doi: 10.1016/j.cj.2020.12.010
[44] OKPALA N E, POTCHO M P, IMRAN M, et al. Starch morphology and metabolomic analyses reveal that the effect of high temperature on cooked rice elongation and expansion varied in indica and japonica rice cultivars[J]. Agronomy, 2021, 11(12): 2416. doi: 10.3390/agronomy11122416
[45] POTCHO P M, OKPALA N E, KOROHOU T, et al. Nitrogen sources affected the biosynthesis of 2-acetyl-1-pyrroline, cooked rice elongation and amylose content in rice[J]. PLoS One, 2021, 16(7): e254182.
[46] AB HALIM A A B, RAFII M Y, OSMAN M B, et al. Ageing effects, generation means, and path coefficient analyses on high kernel elongation in Mahsuri Mutan and Basmati 370 rice populations[J]. Biomed Research International, 2021, 2021: 8350136. doi: 10.1155/2021/8350136
[47] 徐伟清, 王小雷, 刘杨, 等. 稻米蒸煮特性QTL定位及与感官食味品质的相关性分析[J]. 核农学报, 2022, 36(1): 66-74. doi: 10.11869/j.issn.100-8551.2022.01.0066 [48] 刘宜柏, 黄英金. 稻米食味品质的相关性研究[J]. 江西农业大学学报, 1989, 11(4): 1-5. doi: 10.13836/j.jjau.1989050 [49] AHN S N, BOLLICH C N, MCCLUNG A M, et al. RFLP analysis of genomic regions associated with cooked-kernel elongation in rice[J]. Theoretical and Applied Genetics, 1993, 87(1/2): 27-32.
[50] SANTOS M V, CUEVAS R P O, SREENIVASULU N, et al. Measurement of rice grain dimensions and chalkiness, and rice grain elongation using image analysis[J]. Methods in Molecular Biology, 2019, 1892: 99-108.
[51] SCHNEIDER C A, RASBAND W S, ELICEIRI K W. NIH Image to ImageJ: 25 years of image analysis[J]. Nature methods, 2012, 9(7): 671-675. doi: 10.1038/nmeth.2089
[52] JINOROSE M, PRACHAYAWARAKORN S, SOPONRONNARIT S. A novel image-analysis based approach to evaluate some physicochemical and cooking properties of rice kernels[J]. Journal of Food Engineering, 2014, 124: 184-190.
[53] SUMAN K, MADHUBABU P, RATHOD R, et al. Variation of grain quality characters and marker-trait association in rice (Oryza sativa L.)[J]. Journal of Genetics, 2020, 99(1): 5. doi: 10.1007/s12041-019-1164-4
[54] 黄发松, 孙宗修, 胡培松, 等. 食用稻米品质形成研究的现状与展望[J]. 中国水稻科学, 1998, 12(3): 172-176. doi: 10.3321/j.issn:1001-7216.1998.03.012 [55] JIANG Y, CHEN Y, ZHAO C, et al. The starch physicochemical properties between superior and inferior grains of japonica rice under panicle nitrogen fertilizer determine the difference in eating quality[J]. Foods, 2022, 11(16): 2489. doi: 10.3390/foods11162489
[56] TESTER R F, KARKALAS J, QI X. Starch-composition, fine structure and architecture[J]. Journal of Cereal Science, 2004, 39(2): 151-165. doi: 10.1016/j.jcs.2003.12.001
[57] RAIGOND P, EZEKIEL R, RAIGOND B. Resistant starch in food: A review[J]. Journal of the Science of Food and Agriculture, 2015, 95(10): 1968-1978. doi: 10.1002/jsfa.6966
[58] WEI C, QIN F, ZHOU W, et al. Comparison of the crystalline properties and structural changes of starches from high-amylose transgenic rice and its wild type during heating[J]. Food Chemistry, 2011, 128(3): 645-652. doi: 10.1016/j.foodchem.2011.03.080
[59] ZHOU H, WANG L, LIU G, et al. Critical roles of soluble starch synthase SSIIIa and granule-bound starch synthase Waxy in synthesizing resistant starch in rice[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(45): 12844-12849.
[60] PAN T, LIN L, ZHANG L, et al. Changes in kernel properties, in situ gelatinization, and physicochemical properties of waxy rice with inhibition of starch branching enzyme during cooking[J]. International Journal of Food Science and Technology, 2019, 54(9): 2780-2791. doi: 10.1111/ijfs.14193
[61] HE W, LIN L, WANG J, et al. Inhibition of starch branching enzymes in waxy rice increases the proportion of long branch-chains of amylopectin resulting in the comb-like profiles of starch granules[J]. Plant Science, 2018, 277: 177-187.
[62] SHI S, PAN K, ZHANG G, et al. Differences in grain protein content and regional distribution of 706 rice accessions[J]. Journal of the Science of Food and Agriculture, 2023, 103(3): 1593-1599. doi: 10.1002/jsfa.12308
[63] KUMAR P, PRAKASH K S, JAN K, et al. Effects of gamma irradiation on starch granule structure and physicochemical properties of brown rice starch[J]. Journal of Cereal Science, 2017, 77: 194-200. doi: 10.1016/j.jcs.2017.08.017
[64] CHAMPAGNE E T, BETT-GARBER K L, THOMSON J L, et al. Unraveling the impact of nitrogen nutrition on cooked rice flavor and texture[J]. Cereal Chemistry Journal, 2009, 86(3): 274-280. doi: 10.1094/CCHEM-86-3-0274
[65] LYON B G, CHAMPAGNE E T, VINYARD B T, et al. Effects of degree of milling, drying condition, and final moisture content on sensory texture of cooked rice[J]. Cereal Chemistry, 1999, 76(1): 56-62. doi: 10.1094/CCHEM.1999.76.1.56
[66] SHI S, ZHANG G, CHEN L, et al. Different nitrogen fertilizer application in the field affects the morphology and structure of protein and starch in rice during cooking[J]. Food Research International, 2023, 163: 112193. doi: 10.1016/j.foodres.2022.112193
[67] BALINDONG J L, WARD R M, LIU L, et al. Rice grain protein composition influences instrumental measures of rice cooking and eating quality[J]. Journal of Cereal Science, 2018, 79: 35-42. doi: 10.1016/j.jcs.2017.09.008
[68] 习敏, 季雅岚, 文革, 等. 水稻食味品质形成影响因素研究与展望[J]. 中国农学通报, 2020, 36(12): 159-164. [69] SHI S, ZHANG G, ZHAO D, et al. Changes in water absorption and morphology of rice with different eating quality during soaking[J]. European Food Research and Technology, 2023, 249(3): 759-766. doi: 10.1007/s00217-022-04173-x
[70] 张栋昊, 蔡妍培, 劳菲, 等. 大米蛋白质与米饭食味品质关联性研究进展[J]. 食品科学, 2022, 44(9): 270-277. [71] ZHAN Q, YE X, ZHANG Y, et al. Starch granule-associated proteins affect the physicochemical properties of rice starch[J]. Food Hydrocolloids, 2020, 101: 105504. doi: 10.1016/j.foodhyd.2019.105504.
[72] HU Z, YANG Y, LU L, et al. Kinetics of water absorption expansion of rice during soaking at different temperatures and correlation analysis upon the influential factors[J]. Food Chemistry, 2021, 346: 128912. doi: 10.1016/j.foodchem.2020.128912
[73] SHI J, WU M, QUAN M. Effects of protein oxidation on gelatinization characteristics during rice storage[J]. Journal of Cereal Science, 2017, 75: 228-233. doi: 10.1016/j.jcs.2017.04.013
[74] YANG W, XU P, ZHANG J, et al. OsbZIP60-mediated unfolded protein response regulates grain chalkiness in rice[J]. Journal of Genetics and Genomics, 2022, 49(5): 414-426. doi: 10.1016/j.jgg.2022.02.002
[75] 廖斌, 张桂莲. 水稻垩白的研究进展[J]. 作物研究, 2015, 29(1): 77-83. doi: 10.3969/j.issn.1001-5280.2015.01.20 [76] LI Y, FAN C, XING Y, et al. Chalk5 encodes a vacuolar H+-translocating pyrophosphatase influencing grain chalkiness in rice[J]. Nature Genetics, 2014, 46(4): 398-404. doi: 10.1038/ng.2923
[77] SINGH N, SODHI N S, KAUR M, et al. Physico-chemical, morphological, thermal, cooking and textural properties of chalky and translucent rice kernels[J]. Food Chemistry, 2003, 82(3): 433-439. doi: 10.1016/S0308-8146(03)00007-4
[78] CHENG F M, ZHONG L J, WANG F, et al. Differences in cooking and eating properties between chalky and translucent parts in rice grains[J]. Food Chemistry, 2005, 90(1/2): 39-46.
[79] CHUN A, SONG J, KIM K, et al. Quality of head and chalky rice and deterioration of eating quality by chalky rice[J]. Journal of Crop Science and Biotechnology, 2009, 12(4): 239-244. doi: 10.1007/s12892-009-0142-4
[80] 王忠, 顾蕴洁, 陈刚, 等. 稻米的品质和影响因素[J]. 分子植物育种, 2003, 1(2): 231-241. doi: 10.3969/j.issn.1672-416X.2003.02.011 [81] 卢林, 孙成效, 朱智伟, 等. 我国稻米品质标准及检测技术创新概述[J]. 中国稻米, 2022, 28(1): 1-6. [82] TONG C, GAO H, LUO S, et al. Impact of postharvest operations on rice grain quality: A review[J]. Comprehensive Reviews in Food Science and Food Safety, 2019, 18(3): 626-640. doi: 10.1111/1541-4337.12439
[83] 郭桂英, 王青林, 马汉云, 等. 碾磨品质对籼稻食味品质的影响[J]. 天津农业科学, 2017, 23(6): 40-44. [84] KIM S Y, LEE H. Effects of eating quality on milled rice produced from brown rice with different milling conditions[J]. Journal of the Korean Society for Applied Biological Chemistry, 2013, 56(5): 621-629. doi: 10.1007/s13765-013-3097-6
[85] MOHAPATRA D, BAL S. Cooking quality and instrumental textural attributes of cooked rice for different milling fractions[J]. Journal of Food Engineering, 2006, 73(3): 253-259. doi: 10.1016/j.jfoodeng.2005.01.028
[86] YANG X, BI J, GILBERT R G, et al. Amylopectin chain length distribution in grains of japonica rice as affected by nitrogen fertilizer and genotype[J]. Journal of Cereal Science, 2016, 71: 230-238. doi: 10.1016/j.jcs.2016.09.003
[87] JULIANO B O. Physico-chemical properties of starch and protein and their relation to grain quality and nutritional value of rice[J]. Rice Breeding, 1972, 5: 389-405.
[88] 袁玉洁, 张丝琪, 王明玥, 等. 蒸煮米水比对不同直链淀粉含量杂交籼稻米粒微观结构和食味特性的影响[J]. 作物学报, 2022, 48(12): 3225-3233. [89] 李萍, 周广春, 崔晶, 等. 煮饭水质结合加水量和浸泡时间对粳稻食味的影响[J]. 中国稻米, 2021, 27(6): 74-79. doi: 10.3969/j.issn.1006-8082.2021.06.015 [90] HUSSIAN R A, BROWN D C. Use of two-dimensional grid patterns to limit hazardous ambulation in demented patients[J]. Journal of Gerontology, 1987, 42(5): 558-560. doi: 10.1093/geronj/42.5.558
[91] 高振宇, 曾大力, 崔霞, 等. 水稻稻米糊化温度控制基因ALK的图位克隆及其序列分析[J]. 中国科学(C辑: 生命科学), 2003, 33(6): 481-487. [92] 张桂权. 基于SSSL文库的水稻设计育种平台[J]. 遗传, 2019, 41(8): 754-760. doi: 10.16288/j.yczz.19-105 [93] ZHANG G. Target chromosome-segment substitution: A way to breeding by design in rice[J]. The Crop Journal, 2021, 9(3): 658-668. doi: 10.1016/j.cj.2021.03.001
-
期刊类型引用(1)
1. 周光亮,许源峰,杨慧,李新云,赵云翔,刘向东. 全产业链猪育种体系构建的研究进展. 中国猪业. 2024(03): 59-67 .  百度学术
百度学术
其他类型引用(0)




 下载:
下载: